Introductory Topics - AP Chemistry
Card 0 of 900
How many of the following compounds are ionic?





How many of the following compounds are ionic?
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.
 contains a positively charged
contains a positively charged  ion and negatively charged
ion and negatively charged 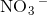 ion which form an ionic bond.
ion which form an ionic bond.  contains two positively charged
contains two positively charged  ions and a negatively charged
ions and a negatively charged  ion which form an ionic bond.
ion which form an ionic bond. 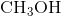 ,
,  , and
, and  are all examples of molecules that contain covalent bonds. None of them contains any charged ions. The answer is therefore "two,"referring to the
are all examples of molecules that contain covalent bonds. None of them contains any charged ions. The answer is therefore "two,"referring to the  and
and  molecules.
molecules.
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.


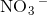



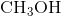




Compare your answer with the correct one above
What is the molecular geometry of  ?
?
What is the molecular geometry of 
Start by drawing the Lewis structure of  .
.

Selenium has  fluoride molecules bonded to it, and it has a lone pair. This means its steric number is
fluoride molecules bonded to it, and it has a lone pair. This means its steric number is  . A molecule with a steric number of
. A molecule with a steric number of  and
and  lone pair has a seesaw molecular geometry. Note that this is an expanded octet.
lone pair has a seesaw molecular geometry. Note that this is an expanded octet.
Start by drawing the Lewis structure of 

Selenium has 



Compare your answer with the correct one above
What is the molecular geometry of  ?
?
What is the molecular geometry of 
Start by drawing the Lewis structure of  .
.

Notice that the central oxygen atom has  other oxygen atoms bonded to it, as well as a lone pair. This means it has a steric number of
other oxygen atoms bonded to it, as well as a lone pair. This means it has a steric number of  . A molecule with a steric number of
. A molecule with a steric number of  and
and  lone pair has a bent molecular geometry.
lone pair has a bent molecular geometry.
Start by drawing the Lewis structure of 

Notice that the central oxygen atom has 



Compare your answer with the correct one above
What is the molecular geometry of the bonds surrounding the circled atom?

What is the molecular geometry of the bonds surrounding the circled atom?


Start by determining the steric number of the circled oxygen atom. Since it is bonded to a hydrogen and a carbon and has  lone pairs, its steric number must be
lone pairs, its steric number must be  . A molecule with a steric number of
. A molecule with a steric number of  and
and  lone pairs has a bent molecular geometry.
lone pairs has a bent molecular geometry.

Start by determining the steric number of the circled oxygen atom. Since it is bonded to a hydrogen and a carbon and has 



Compare your answer with the correct one above
Which of the following correctly describes the VSEPR shape of the water molecule,  ?
?
Which of the following correctly describes the VSEPR shape of the water molecule, 
In the water molecule, there are four electron groups (steric number is four). Two of them (two pairs) are bonded (the bonds between both hydrogen atoms and the oxygen atom), while the other two are lone pairs on oxygen. Thus, the water molecule has a bent shape, with two bonded electron groups and two lone pair electron groups. A linear VSEPR shape has two electron groups, a trigonal planar has three electron groups, and a trigonal bipyramidal shape has five electron groups.
In the water molecule, there are four electron groups (steric number is four). Two of them (two pairs) are bonded (the bonds between both hydrogen atoms and the oxygen atom), while the other two are lone pairs on oxygen. Thus, the water molecule has a bent shape, with two bonded electron groups and two lone pair electron groups. A linear VSEPR shape has two electron groups, a trigonal planar has three electron groups, and a trigonal bipyramidal shape has five electron groups.
Compare your answer with the correct one above
What is the molecular geometry of the circled element?

What is the molecular geometry of the circled element?

In order to figure out the molecular geometry of the circled element, first determine the steric number.
The circled nitrogen has three electron groups attached to it, so its steric number is  . Since there are no lone pairs on the nitrogen, its electron geometry and its molecular geometry are both trigonal planar.
. Since there are no lone pairs on the nitrogen, its electron geometry and its molecular geometry are both trigonal planar.
In order to figure out the molecular geometry of the circled element, first determine the steric number.
The circled nitrogen has three electron groups attached to it, so its steric number is 
Compare your answer with the correct one above
What is the molecular geometry of the circled element?

What is the molecular geometry of the circled element?

In order to figure out the molecular geometry of the circled element, first determine the steric number.
Since the circled oxygen has four electron groups attached to it, its steric number is  . The circled oxygen also has
. The circled oxygen also has  lone pairs of electrons attached to it; therefore, it has a bent molecular geometry.
lone pairs of electrons attached to it; therefore, it has a bent molecular geometry.
In order to figure out the molecular geometry of the circled element, first determine the steric number.
Since the circled oxygen has four electron groups attached to it, its steric number is 

Compare your answer with the correct one above
Which of the following is true of Valence Bond theory and Molecular Orbital theory?
Which of the following is true of Valence Bond theory and Molecular Orbital theory?
The Valence Bond theory states that covalent bonds are formed from atomic orbital overlap while the Molecular Orbital theory is the mathematical combination of atomic orbitals to produce anti bonding and bonding orbitals.
Hybridization occurs through combining atomic orbitals, a concept consistent with Valence Bond theory. Common bonds found in Valence Bond hybridization are sigma and pi bonds which overlap end-to-end or side-to-side respectively. Orbitals are combined in Molecular Orbital theory, producing either bonding or anti bonding orbitals. Molecular Orbital theory shows the destructive or constructive interference of sigma and pi bonds (displayed through bonding and anti bonding orbitals).
The Valence Bond theory states that covalent bonds are formed from atomic orbital overlap while the Molecular Orbital theory is the mathematical combination of atomic orbitals to produce anti bonding and bonding orbitals.
Hybridization occurs through combining atomic orbitals, a concept consistent with Valence Bond theory. Common bonds found in Valence Bond hybridization are sigma and pi bonds which overlap end-to-end or side-to-side respectively. Orbitals are combined in Molecular Orbital theory, producing either bonding or anti bonding orbitals. Molecular Orbital theory shows the destructive or constructive interference of sigma and pi bonds (displayed through bonding and anti bonding orbitals).
Compare your answer with the correct one above
A molecule has 4 electron groups and 2 lone pairs. What is the VSEPR notation, electron group geometry, and molecular geometry?
A molecule has 4 electron groups and 2 lone pairs. What is the VSEPR notation, electron group geometry, and molecular geometry?
A molecule that has 4 electron groups and 2 lone pairs has an VSEPR notation of: . The
. The  indicates the central atom, the
indicates the central atom, the  the number of bonding atoms, and
the number of bonding atoms, and  the number of lone pairs. There are 2 bonding atoms and 2 lone pairs, totaling a total of 4 electron groups. All tetrahedral geometries consist of 4 electron groups. Molecular geometries of electron groups with the tetrahedral are tetrahedral (
the number of lone pairs. There are 2 bonding atoms and 2 lone pairs, totaling a total of 4 electron groups. All tetrahedral geometries consist of 4 electron groups. Molecular geometries of electron groups with the tetrahedral are tetrahedral ( ), trigonal pyramidal (
), trigonal pyramidal ( ), and bent (
), and bent ( ).
).
Therefore the correct answer is:  , tetrahedral, bent.
, tetrahedral, bent.
A molecule that has 4 electron groups and 2 lone pairs has an VSEPR notation of:






Therefore the correct answer is: 
Compare your answer with the correct one above
What is the molecular geometry of the molecule  ?
?
What is the molecular geometry of the molecule 
 is t-shaped. By drawing the Lewis diagram for
is t-shaped. By drawing the Lewis diagram for  , we see that there are 5 electron groups and 2 lone pairs on the central atom,
, we see that there are 5 electron groups and 2 lone pairs on the central atom,  . Electron group geometry states that molecules with 5 electron groups have a trigonal bipyramidal geometry. The molecular geometries of trigonal bipyramidal molecules can be trigonal bipyramidal (no lone pairs), seesaw (1 lone pair), t-shaped (2 lone pairs). and linear (3 lone pairs).
. Electron group geometry states that molecules with 5 electron groups have a trigonal bipyramidal geometry. The molecular geometries of trigonal bipyramidal molecules can be trigonal bipyramidal (no lone pairs), seesaw (1 lone pair), t-shaped (2 lone pairs). and linear (3 lone pairs).
 consists of 5 electron groups and 2 lone pairs and therefore fits the molecular geometry of a t-shaped molecule.
consists of 5 electron groups and 2 lone pairs and therefore fits the molecular geometry of a t-shaped molecule.




Compare your answer with the correct one above
Which of the following is true of both molecular orbitals and atomic orbitals?
Which of the following is true of both molecular orbitals and atomic orbitals?
The number of molecular orbitals is equal to the number of atomic orbitals for a given molecule. The number of orbitals is consistent. Molecular orbitals shows destructive and constructive interference between sigma and pi bonds. In molecular orbital diagrams, these results appear as either bonding or anti bonding energies. Atomic orbitals combine the valence shells of overlapping orbitals to form more stable electron configurations.
The number of molecular orbitals is equal to the number of atomic orbitals for a given molecule. The number of orbitals is consistent. Molecular orbitals shows destructive and constructive interference between sigma and pi bonds. In molecular orbital diagrams, these results appear as either bonding or anti bonding energies. Atomic orbitals combine the valence shells of overlapping orbitals to form more stable electron configurations.
Compare your answer with the correct one above
What is the hybridization of a  molecule?
molecule?
What is the hybridization of a 
The correct hybridization of a  is
is  (trigonal bipyramidal). By drawing a Lewis diagram for a
(trigonal bipyramidal). By drawing a Lewis diagram for a  molecule, we find 5 electron groups. Therefore,
molecule, we find 5 electron groups. Therefore,  .
.
The correct hybridization of a 



Compare your answer with the correct one above
The unique properties of water, namely its incredibly high heat capacity and surface tension, can be attributed to which of the following kinds of intermolecular/intramolecular forces?
The unique properties of water, namely its incredibly high heat capacity and surface tension, can be attributed to which of the following kinds of intermolecular/intramolecular forces?
The partial negative and positive charges on a water molecule allow it to be attracted to other polar water molecules, which creates the cohesive nature of water and contributes to its high surface tension and heat capacity. London dispersion and Van der Waals forces are significantly weaker than hydrogen bonding. Additionally, ionic bonding only occurs intramolecularly, so it has little effect on the intermolecular properties of water.
The partial negative and positive charges on a water molecule allow it to be attracted to other polar water molecules, which creates the cohesive nature of water and contributes to its high surface tension and heat capacity. London dispersion and Van der Waals forces are significantly weaker than hydrogen bonding. Additionally, ionic bonding only occurs intramolecularly, so it has little effect on the intermolecular properties of water.
Compare your answer with the correct one above
Which of the following is not true about water?
Which of the following is not true about water?
Unlike most other solvents, water is unique in that its solid form is actually less dense than its liquid form. This is precisely the reason why ice floats in water. Water does indeed participate in many biochemical reactions and can form hydrogen bonds with other water molecules to allow for great cohesion. Also, the specific heat capacity of water is  , which is the amount of heat required to raise the temperature of one mole of substance by 1 Kelvin. This value is much higher than the specific heat capacities of many other solvents. For example, the specific heat capacity for hexane is
, which is the amount of heat required to raise the temperature of one mole of substance by 1 Kelvin. This value is much higher than the specific heat capacities of many other solvents. For example, the specific heat capacity for hexane is  .
.
Unlike most other solvents, water is unique in that its solid form is actually less dense than its liquid form. This is precisely the reason why ice floats in water. Water does indeed participate in many biochemical reactions and can form hydrogen bonds with other water molecules to allow for great cohesion. Also, the specific heat capacity of water is 

Compare your answer with the correct one above
Is water an acid or a base?
Is water an acid or a base?
Water is an amphipathic molecule, meaning that it can act as both an acid and a base. In some situations, water can act as a Bronsted-Lowry base (defined as a species which accepts a proton). Here is an example of water acting as a base:

As you can see,  accepted a hydrogen atom (proton) to become
accepted a hydrogen atom (proton) to become  .
.
In other situations, water can act as a Bronsted-Lowry base (defined as a species which donates a proton). Here is an example of water acting as an acid:

As you can see,  donated a hydrogen atom (proton) to become
donated a hydrogen atom (proton) to become  .
.
Thus, water can act as both an acid and a base.
Water is an amphipathic molecule, meaning that it can act as both an acid and a base. In some situations, water can act as a Bronsted-Lowry base (defined as a species which accepts a proton). Here is an example of water acting as a base:
As you can see, 

In other situations, water can act as a Bronsted-Lowry base (defined as a species which donates a proton). Here is an example of water acting as an acid:
As you can see, 

Thus, water can act as both an acid and a base.
Compare your answer with the correct one above
Water is said to be the solvent of life due to its very unique properties. Which of the following does not represent one of the features of water that gives it uniqueness?
Water is said to be the solvent of life due to its very unique properties. Which of the following does not represent one of the features of water that gives it uniqueness?
In this question, we're asked to identify an answer choice that describes something that is not a characteristic of water.
Remember that water molecules consist of a single oxygen atom with single bonds to two hydrogen atoms. There are also two lone pairs of electrons on the oxygen atom, giving the overall molecular structure of water a bent shape. This bent shape is important, because it results in an overall net dipole moment of the water molecule, where the two hydrogen atoms have a partial positive charge and the oxygen atom has a partial negative charge.
The significance of this partial charge separation is that it allows water to be excellent at forming hydrogen bonds. Recall that hydrogen bonds occur when a partially positive hydrogen atom participates in a non-covalent bond to another electronegative atom on some other molecule (though can also be intramolecular in bigger compounds, like enzymes). These intermolecular interactions between individual water molecules results in an overall strong force of attraction between them.
As a consequence of these strong hydrogen bonds between the water molecules, many unique properties of water result. For instance, liquid water has an unusually high heat capacity. Due to the strong intermolecular forces of attraction, it takes a relatively large amount of energy in order overcome the attractive forces that hold water molecules together. Another very unique property of water is that its solid phase is actually less dense than its liquid phase. Thus, when water freezes into ice, it will float to the top surface in a body of water, which is important for sustaining life in certain environments.
Finally, these strong intermolecular hydrogen bonds give water a high surface tension. Once again, the reason for this is due to the fact that it takes a relatively greater amount of energy in order to break these bonds apart. Thus, water does not have low surface tension, making this the correct choice.
In this question, we're asked to identify an answer choice that describes something that is not a characteristic of water.
Remember that water molecules consist of a single oxygen atom with single bonds to two hydrogen atoms. There are also two lone pairs of electrons on the oxygen atom, giving the overall molecular structure of water a bent shape. This bent shape is important, because it results in an overall net dipole moment of the water molecule, where the two hydrogen atoms have a partial positive charge and the oxygen atom has a partial negative charge.
The significance of this partial charge separation is that it allows water to be excellent at forming hydrogen bonds. Recall that hydrogen bonds occur when a partially positive hydrogen atom participates in a non-covalent bond to another electronegative atom on some other molecule (though can also be intramolecular in bigger compounds, like enzymes). These intermolecular interactions between individual water molecules results in an overall strong force of attraction between them.
As a consequence of these strong hydrogen bonds between the water molecules, many unique properties of water result. For instance, liquid water has an unusually high heat capacity. Due to the strong intermolecular forces of attraction, it takes a relatively large amount of energy in order overcome the attractive forces that hold water molecules together. Another very unique property of water is that its solid phase is actually less dense than its liquid phase. Thus, when water freezes into ice, it will float to the top surface in a body of water, which is important for sustaining life in certain environments.
Finally, these strong intermolecular hydrogen bonds give water a high surface tension. Once again, the reason for this is due to the fact that it takes a relatively greater amount of energy in order to break these bonds apart. Thus, water does not have low surface tension, making this the correct choice.
Compare your answer with the correct one above
Which of the following substances is the least soluble in water?
Which of the following substances is the least soluble in water?
 (benzene) is the least soluble in water because it is a non polar substance. Polar compounds are soluble in water because like dissolves like. Water is polar and therefore dissolves other polar substances. The more polar or ionic a substance is, the more soluble it is in water.
(benzene) is the least soluble in water because it is a non polar substance. Polar compounds are soluble in water because like dissolves like. Water is polar and therefore dissolves other polar substances. The more polar or ionic a substance is, the more soluble it is in water.  ,
,  ,
,  are polar compounds because of the uneven electron distribution within these compounds.
are polar compounds because of the uneven electron distribution within these compounds.




Compare your answer with the correct one above
In an ionic bond, electrons are .
In an ionic bond, electrons are .
In an ionic bond, electrons are transferred to make a compound. For example, a sodium atom and a chloride atom can combine, exchanging electrons to form:  and
and 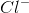 in solution, or
in solution, or  (sodium chloride/table salt). Electrons are shared only in covalent bonds. Electrons can be repelled if two (or more) electrons are within very close proximity of one another. Electrons can be excited by absorbing energy and then subsequently jumping from the ground state to a higher, less stable state. Electrons may never be destroyed and/or created.
(sodium chloride/table salt). Electrons are shared only in covalent bonds. Electrons can be repelled if two (or more) electrons are within very close proximity of one another. Electrons can be excited by absorbing energy and then subsequently jumping from the ground state to a higher, less stable state. Electrons may never be destroyed and/or created.
In an ionic bond, electrons are transferred to make a compound. For example, a sodium atom and a chloride atom can combine, exchanging electrons to form: 
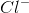

Compare your answer with the correct one above
Which of these can be formed when ionic bonds break down?
Which of these can be formed when ionic bonds break down?
Ionic bonds are bonds involving the attraction between oppositely-charged ions. An example of an ionic compound is  , or table salt. The sodium is positively-charged, while the chlorine is negatively-charged. These opposite charges attract via an ionic bond to form the ionic compound
, or table salt. The sodium is positively-charged, while the chlorine is negatively-charged. These opposite charges attract via an ionic bond to form the ionic compound  . When this ionic bond breaks, the sodium and chlorine separate into ions:
. When this ionic bond breaks, the sodium and chlorine separate into ions:  (the positively-charged cation) and
(the positively-charged cation) and 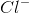 (the negatively-charged anion).
(the negatively-charged anion).
Ionic compounds have a characteristic lattice structure--that is, the arrangement of ions in a regular, geometric pattern. In the case of  , this structure simply refers to the arrangement of
, this structure simply refers to the arrangement of  and
and  ions in a pattern to form
ions in a pattern to form  . It forms when an ionic compound is being created, not broken down.
. It forms when an ionic compound is being created, not broken down.
Lastly, micelles are lipid molecules which, in aqueous solution, arrange themselves in a spherical form. This is a response to the fact that fatty acids have both hydrophilic and hydrophobic regions (they are amphipathic). It has nothing to do with ionic bonds.
Ionic bonds are bonds involving the attraction between oppositely-charged ions. An example of an ionic compound is 


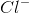
Ionic compounds have a characteristic lattice structure--that is, the arrangement of ions in a regular, geometric pattern. In the case of 



Lastly, micelles are lipid molecules which, in aqueous solution, arrange themselves in a spherical form. This is a response to the fact that fatty acids have both hydrophilic and hydrophobic regions (they are amphipathic). It has nothing to do with ionic bonds.
Compare your answer with the correct one above
What is the charge on the cation in the ionic compound sodium oxide?
What is the charge on the cation in the ionic compound sodium oxide?
When sodium and oxygen form the ionic compound sodium oxide,  , each sodium ion transfers an electron to the central oxygen atom. Thus, each sodium atom becomes a positive ion (or cation) with a charge of +1.
, each sodium ion transfers an electron to the central oxygen atom. Thus, each sodium atom becomes a positive ion (or cation) with a charge of +1.
When sodium and oxygen form the ionic compound sodium oxide, 
Compare your answer with the correct one above




