Non-Ideal Gas Behavior - AP Chemistry
Card 0 of 8
One flask is at STP and another is at  . What is the pressure at
. What is the pressure at 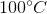 ?
?
One flask is at STP and another is at 
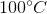
The pressure of the flask at  is
is  .
.

Because the volume between the flasks and the moles in each flask are constant, we can cancel out  and
and  .
.

At STP, conditions are  and
and  .
.
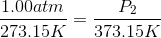

The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Compare your answer with the correct one above
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.


A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.
Recall the van der Waals equation for non-ideal gases
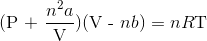
Rewrite the equation using the known values and solve for P
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)


Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Compare your answer with the correct one above
One flask is at STP and another is at  . What is the pressure at
. What is the pressure at 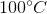 ?
?
One flask is at STP and another is at 
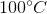
The pressure of the flask at  is
is  .
.

Because the volume between the flasks and the moles in each flask are constant, we can cancel out  and
and  .
.

At STP, conditions are  and
and  .
.
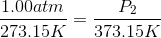

The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Compare your answer with the correct one above
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.


A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.
Recall the van der Waals equation for non-ideal gases
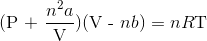
Rewrite the equation using the known values and solve for P
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)


Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Compare your answer with the correct one above
One flask is at STP and another is at  . What is the pressure at
. What is the pressure at 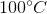 ?
?
One flask is at STP and another is at 
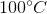
The pressure of the flask at  is
is  .
.

Because the volume between the flasks and the moles in each flask are constant, we can cancel out  and
and  .
.

At STP, conditions are  and
and  .
.
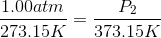

The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Compare your answer with the correct one above
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.


A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.
Recall the van der Waals equation for non-ideal gases
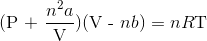
Rewrite the equation using the known values and solve for P
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)


Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Compare your answer with the correct one above
One flask is at STP and another is at  . What is the pressure at
. What is the pressure at 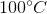 ?
?
One flask is at STP and another is at 
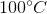
The pressure of the flask at  is
is  .
.

Because the volume between the flasks and the moles in each flask are constant, we can cancel out  and
and  .
.

At STP, conditions are  and
and  .
.
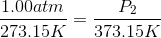

The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Compare your answer with the correct one above
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.


A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.
Recall the van der Waals equation for non-ideal gases
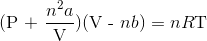
Rewrite the equation using the known values and solve for P
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)


Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Compare your answer with the correct one above