Phases of Matter - AP Chemistry
Card 0 of 276
Distillation requires which of the following?
Distillation requires which of the following?
Distillation is the process by which liquids are purified of impurities. Distillation first requires vaporization of liquids to become pure gases. The gases are then cooled and turned back into pure liquids via condensation into a separate container. A good distillation will remove all impurities from the liquid. This is why distilled water is used for chemical solutions; it does not contain ions or other impurities that could interfere with reaction.
Distillation is the process by which liquids are purified of impurities. Distillation first requires vaporization of liquids to become pure gases. The gases are then cooled and turned back into pure liquids via condensation into a separate container. A good distillation will remove all impurities from the liquid. This is why distilled water is used for chemical solutions; it does not contain ions or other impurities that could interfere with reaction.
Compare your answer with the correct one above
Which of the following occurs during vaporization?
Which of the following occurs during vaporization?
Vaporization refers to the phase change from liquid to gas, also known as evaporation. When becoming a gas, atoms spread out and expand to fill whatever container they are in. Conservation of mass hold that atoms are never created nor destroyed. Atoms becoming more organized and forming a more rigid shape describes a phase change toward becoming solid.
Vaporization refers to the phase change from liquid to gas, also known as evaporation. When becoming a gas, atoms spread out and expand to fill whatever container they are in. Conservation of mass hold that atoms are never created nor destroyed. Atoms becoming more organized and forming a more rigid shape describes a phase change toward becoming solid.
Compare your answer with the correct one above
Which of the following is a property of crystalline solids?
Which of the following is a property of crystalline solids?
A crystalline solid is made up of a repeating pattern of atoms called a crystal lattice. Atoms moving about randomly, and taking the shape of their container are descriptions of fluids (liquids and gasses).
A crystalline solid is made up of a repeating pattern of atoms called a crystal lattice. Atoms moving about randomly, and taking the shape of their container are descriptions of fluids (liquids and gasses).
Compare your answer with the correct one above
Which of the following properties will decrease as a solute is dissolved in a liquid?
Which of the following properties will decrease as a solute is dissolved in a liquid?
Dissolving a solute in a solvent increases the number of dissolved particles per mass of solvent. Freezing point depression is a colligative property of solutions, and will decrease as the molality of the solution increases. Boiling point and osmotic pressure increase as the molality of the solution increases. Temperature is unaffected by the dissolution of solutes in a solvent.
Dissolving a solute in a solvent increases the number of dissolved particles per mass of solvent. Freezing point depression is a colligative property of solutions, and will decrease as the molality of the solution increases. Boiling point and osmotic pressure increase as the molality of the solution increases. Temperature is unaffected by the dissolution of solutes in a solvent.
Compare your answer with the correct one above
Which of the following is a property of a solid?
Which of the following is a property of a solid?
Solids are defined as having definite shapes and volumes. The atoms in a solid touch one another and are fixed in rigid positions. Unlike liquids or gases, molecules inside a solid are not able to freely move past one another. Solids will retain their shape even when put in open atmospheres.
Solids are defined as having definite shapes and volumes. The atoms in a solid touch one another and are fixed in rigid positions. Unlike liquids or gases, molecules inside a solid are not able to freely move past one another. Solids will retain their shape even when put in open atmospheres.
Compare your answer with the correct one above
Which of the following is not a characteristic of solid elemental aluminum?
Which of the following is not a characteristic of solid elemental aluminum?
Aluminum is not a high-density metal. You may figure this out by comparing aluminum's density (2.7 g/mL) to the density of other metals. Aluminum is very lightweight compared to other metals, making it ideal for the construction of things like airplanes.
Aluminum is a good conductor of heat and electricity. It is ductile, meaning it can be turned into wire, and it is malleable, meaning it can be made into sheets.
Aluminum is not a high-density metal. You may figure this out by comparing aluminum's density (2.7 g/mL) to the density of other metals. Aluminum is very lightweight compared to other metals, making it ideal for the construction of things like airplanes.
Aluminum is a good conductor of heat and electricity. It is ductile, meaning it can be turned into wire, and it is malleable, meaning it can be made into sheets.
Compare your answer with the correct one above
Which of the following statements concerning the properties of solids are true?
Which of the following statements concerning the properties of solids are true?
Solids have well defined shapes because their atoms are held tightly together, only allowing localized movement. Because a solid's atoms are so close together, solids are incompressible. There so little space between atoms that they can't get any closer together. The reason a solid's atoms are so close together is they experience strong intermolecular forces, resulting in high stability. Solids can either be crystalline or amorphous in structure. Crystalline structure is regimented and grid-like, while amorphous has no regular, repeating pattern of atoms.
Solids have well defined shapes because their atoms are held tightly together, only allowing localized movement. Because a solid's atoms are so close together, solids are incompressible. There so little space between atoms that they can't get any closer together. The reason a solid's atoms are so close together is they experience strong intermolecular forces, resulting in high stability. Solids can either be crystalline or amorphous in structure. Crystalline structure is regimented and grid-like, while amorphous has no regular, repeating pattern of atoms.
Compare your answer with the correct one above
Which of the following correctly describes a solid?
Which of the following correctly describes a solid?
A solid is defined as having a definite shape and definite volume. A liquid is defined as having a definite volume, but not a definite shape. A gas is defined as neither having a definite shape nor a definite volume.
A solid is defined as having a definite shape and definite volume. A liquid is defined as having a definite volume, but not a definite shape. A gas is defined as neither having a definite shape nor a definite volume.
Compare your answer with the correct one above
Which of the following best explains why salt is scattered on icy roads and sidewalks?
Which of the following best explains why salt is scattered on icy roads and sidewalks?
The salt decreases the freezing point of the water. Upon addition of salt to icy roads and sidewalks, the ice melts, making it safer to drive and walk. Freezing point depression is a colligative property and depends on the number of dissolved particles, not the type of particles. Thus, sugar would also decrease the freezing point of ice. The amount the freezing point decreases is proportional to the number of dissolved solutes in the solution.
The salt decreases the freezing point of the water. Upon addition of salt to icy roads and sidewalks, the ice melts, making it safer to drive and walk. Freezing point depression is a colligative property and depends on the number of dissolved particles, not the type of particles. Thus, sugar would also decrease the freezing point of ice. The amount the freezing point decreases is proportional to the number of dissolved solutes in the solution.
Compare your answer with the correct one above
Which of the following is a difference between amorphous and crystalline solids?
Which of the following is a difference between amorphous and crystalline solids?
Crystals, or crystalline solids consist of repeated, regular pattern of atoms known as a crystal lattice. Amorphous solids do not have a lattice, and consist of a mass of atoms without a repeated pattern.
Crystals, or crystalline solids consist of repeated, regular pattern of atoms known as a crystal lattice. Amorphous solids do not have a lattice, and consist of a mass of atoms without a repeated pattern.
Compare your answer with the correct one above
The transition from a solid to a gas is known as .
The transition from a solid to a gas is known as .
Some substances will transition from a solid to a gas and skip the liquid phase entirely at standard conditions. This change from a solid to a gas is called sublimation. The reverse process of a gas going to a solid is known as deposition. As an example, solid carbon dioxide (dry ice) will sublimate to produce gaseous carbon dioxide at room temperature.
Evaporation is the process by which a liquid transitions to a gas.
Some substances will transition from a solid to a gas and skip the liquid phase entirely at standard conditions. This change from a solid to a gas is called sublimation. The reverse process of a gas going to a solid is known as deposition. As an example, solid carbon dioxide (dry ice) will sublimate to produce gaseous carbon dioxide at room temperature.
Evaporation is the process by which a liquid transitions to a gas.
Compare your answer with the correct one above
Under which of the following conditions would ice be most likely to sublimate?
Under which of the following conditions would ice be most likely to sublimate?
Sublimation refers to the phase change whereby a substance goes directly from solid to gas. At high temperature and pressure water will be more likely to melt and than evaporate. At low temperature and low pressure, the water will likely stay solid. Likewise at low temperature and high pressure. At high temperature and low pressure, the ice will be most likely to sublimate. This is clear if one looks at the phase diagram for water. High pressure makes it energetically favorable for water to melt before evaporating. Keeping the pressure low, however, makes it more favorable to pass straight into the gaseous phase.
Sublimation refers to the phase change whereby a substance goes directly from solid to gas. At high temperature and pressure water will be more likely to melt and than evaporate. At low temperature and low pressure, the water will likely stay solid. Likewise at low temperature and high pressure. At high temperature and low pressure, the ice will be most likely to sublimate. This is clear if one looks at the phase diagram for water. High pressure makes it energetically favorable for water to melt before evaporating. Keeping the pressure low, however, makes it more favorable to pass straight into the gaseous phase.
Compare your answer with the correct one above
 of an ideal gas are contained in a
of an ideal gas are contained in a  container at a temperature of
container at a temperature of  . The gas exerts a pressure of
. The gas exerts a pressure of  on the container.
on the container.
If pressure is kept constant, what is the final volume of the gas if the temperature of the container is increased to 




If pressure is kept constant, what is the final volume of the gas if the temperature of the container is increased to
Since pressure is kept constant, the only variable that is manipulated is temperature. This means that we can use Charles's law in order to compare volume and temperature. Since volume and temperature are on opposite sides of the ideal gas law, they are directly proportional to one another. As one variable increases, the other will increase as well.
Charles's law is written as follows:

To use this law, we must first convert the temperatures to Kelvin.


Use these temperatures and the initial volume to solve for the final volume.


Since pressure is kept constant, the only variable that is manipulated is temperature. This means that we can use Charles's law in order to compare volume and temperature. Since volume and temperature are on opposite sides of the ideal gas law, they are directly proportional to one another. As one variable increases, the other will increase as well.
Charles's law is written as follows:
To use this law, we must first convert the temperatures to Kelvin.
Use these temperatures and the initial volume to solve for the final volume.
Compare your answer with the correct one above
Which law is the following formula?

Which law is the following formula?
Charles's law defines the direct relationship between temperature and volume. When the parameters of a system change, Charles's law helps us anticipate the effect the changes have on volume and temperature.

Boyle's law relates pressure and volume: 
Charles's law relates temperature and volume: 
Gay-Lussac's law relates temperature and pressure: 
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law: 
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant: 
Charles's law defines the direct relationship between temperature and volume. When the parameters of a system change, Charles's law helps us anticipate the effect the changes have on volume and temperature.
Boyle's law relates pressure and volume:
Charles's law relates temperature and volume:
Gay-Lussac's law relates temperature and pressure:
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law:
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant:
Compare your answer with the correct one above
A canister of gas has a volume of  at
at  . What volume will the gas occupy at
. What volume will the gas occupy at  if the pressure remains constant?
if the pressure remains constant?
A canister of gas has a volume of 


Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:

Our first step to solving this equation will be to convert the given temperatures to Kelvin.


Using these temperatures and the initial volume, we can solve for the final volume of the gas.



Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:
Our first step to solving this equation will be to convert the given temperatures to Kelvin.
Using these temperatures and the initial volume, we can solve for the final volume of the gas.
Compare your answer with the correct one above
A gas occupies a volume of  at
at  . At what temperature, in Kelvin, will the volume of the gas be
. At what temperature, in Kelvin, will the volume of the gas be  ?
?
A gas occupies a volume of 


Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:

Our first step to solving this equation will be to convert the given temperature to Kelvin.

Using this temperature and the given volumes, we can solve for the final temperature of the gas.



Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:
Our first step to solving this equation will be to convert the given temperature to Kelvin.
Using this temperature and the given volumes, we can solve for the final temperature of the gas.
Compare your answer with the correct one above
The graph depicted below represents which of the gas laws?
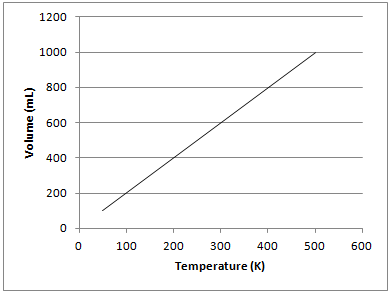
The graph depicted below represents which of the gas laws?
The graph shows that there is a directly proportional relationship between the volume of a gas and temperature in Kelvin when kept at a constant pressure. This is known as Charles’s law and can be represented mathematically as follows:

Gay-Lussac's law shows the relationship between pressure and temperature. Boyle's law shows the relationship between pressure and volume. Newton's third law is not related to gas principles and states that for every force on an object, there is an equal and opposite force of the object on the source of force.
The graph shows that there is a directly proportional relationship between the volume of a gas and temperature in Kelvin when kept at a constant pressure. This is known as Charles’s law and can be represented mathematically as follows:
Gay-Lussac's law shows the relationship between pressure and temperature. Boyle's law shows the relationship between pressure and volume. Newton's third law is not related to gas principles and states that for every force on an object, there is an equal and opposite force of the object on the source of force.
Compare your answer with the correct one above
A balloon filled with room temperature air ( ) has a volume of
) has a volume of  . The balloon is taken outside on a hot summer day where the temperature is
. The balloon is taken outside on a hot summer day where the temperature is  . What will the volume of the balloon be after it is taken outside?
. What will the volume of the balloon be after it is taken outside?
A balloon filled with room temperature air (


We expect the volume to increase since volume and temperature are directly proportional. We know that if we heat something the material will expand so we shouldn't get a value that is smaller than our initial volume. Charles Law says that

where the stuff on the left is the initial volume and temperature and the stuff on the right is the final volume and temperature. First off, we MUST convert the temperatures to Kelvin to use Charles Law. This gives


Solving for the final volume,


We expect the volume to increase since volume and temperature are directly proportional. We know that if we heat something the material will expand so we shouldn't get a value that is smaller than our initial volume. Charles Law says that
where the stuff on the left is the initial volume and temperature and the stuff on the right is the final volume and temperature. First off, we MUST convert the temperatures to Kelvin to use Charles Law. This gives
Solving for the final volume,
Compare your answer with the correct one above
An ideal gas exerts a pressure of  in a
in a  container. The container is at a temperature of
container. The container is at a temperature of  .
.
What will be the final pressure if the volume of the container changes to  ?
?
An ideal gas exerts a pressure of 


What will be the final pressure if the volume of the container changes to 
Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:

Use the given volumes and the initial pressure to solve for the final pressure.


Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:
Use the given volumes and the initial pressure to solve for the final pressure.
Compare your answer with the correct one above
A sample of oxygen gas has a volume of  when its pressure is
when its pressure is  . What will the volume of the gas be at a pressure of
. What will the volume of the gas be at a pressure of  if the temperature remains constant?
if the temperature remains constant?
A sample of oxygen gas has a volume of 


To solve this question we will need to use Boyle's law:

We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.



To solve this question we will need to use Boyle's law:
We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.
Compare your answer with the correct one above