Gases and Gas Laws - AP Chemistry
Card 0 of 172
 of an ideal gas are contained in a
of an ideal gas are contained in a  container at a temperature of
container at a temperature of  . The gas exerts a pressure of
. The gas exerts a pressure of  on the container.
on the container.
If pressure is kept constant, what is the final volume of the gas if the temperature of the container is increased to 




If pressure is kept constant, what is the final volume of the gas if the temperature of the container is increased to
Since pressure is kept constant, the only variable that is manipulated is temperature. This means that we can use Charles's law in order to compare volume and temperature. Since volume and temperature are on opposite sides of the ideal gas law, they are directly proportional to one another. As one variable increases, the other will increase as well.
Charles's law is written as follows:

To use this law, we must first convert the temperatures to Kelvin.


Use these temperatures and the initial volume to solve for the final volume.


Since pressure is kept constant, the only variable that is manipulated is temperature. This means that we can use Charles's law in order to compare volume and temperature. Since volume and temperature are on opposite sides of the ideal gas law, they are directly proportional to one another. As one variable increases, the other will increase as well.
Charles's law is written as follows:
To use this law, we must first convert the temperatures to Kelvin.
Use these temperatures and the initial volume to solve for the final volume.
Compare your answer with the correct one above
Which law is the following formula?

Which law is the following formula?
Charles's law defines the direct relationship between temperature and volume. When the parameters of a system change, Charles's law helps us anticipate the effect the changes have on volume and temperature.

Boyle's law relates pressure and volume: 
Charles's law relates temperature and volume: 
Gay-Lussac's law relates temperature and pressure: 
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law: 
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant: 
Charles's law defines the direct relationship between temperature and volume. When the parameters of a system change, Charles's law helps us anticipate the effect the changes have on volume and temperature.
Boyle's law relates pressure and volume:
Charles's law relates temperature and volume:
Gay-Lussac's law relates temperature and pressure:
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law:
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant:
Compare your answer with the correct one above
A canister of gas has a volume of  at
at  . What volume will the gas occupy at
. What volume will the gas occupy at  if the pressure remains constant?
if the pressure remains constant?
A canister of gas has a volume of 


Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:

Our first step to solving this equation will be to convert the given temperatures to Kelvin.


Using these temperatures and the initial volume, we can solve for the final volume of the gas.



Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:
Our first step to solving this equation will be to convert the given temperatures to Kelvin.
Using these temperatures and the initial volume, we can solve for the final volume of the gas.
Compare your answer with the correct one above
A gas occupies a volume of  at
at  . At what temperature, in Kelvin, will the volume of the gas be
. At what temperature, in Kelvin, will the volume of the gas be  ?
?
A gas occupies a volume of 


Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:

Our first step to solving this equation will be to convert the given temperature to Kelvin.

Using this temperature and the given volumes, we can solve for the final temperature of the gas.



Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:
Our first step to solving this equation will be to convert the given temperature to Kelvin.
Using this temperature and the given volumes, we can solve for the final temperature of the gas.
Compare your answer with the correct one above
The graph depicted below represents which of the gas laws?
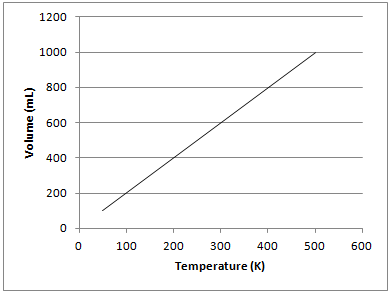
The graph depicted below represents which of the gas laws?
The graph shows that there is a directly proportional relationship between the volume of a gas and temperature in Kelvin when kept at a constant pressure. This is known as Charles’s law and can be represented mathematically as follows:

Gay-Lussac's law shows the relationship between pressure and temperature. Boyle's law shows the relationship between pressure and volume. Newton's third law is not related to gas principles and states that for every force on an object, there is an equal and opposite force of the object on the source of force.
The graph shows that there is a directly proportional relationship between the volume of a gas and temperature in Kelvin when kept at a constant pressure. This is known as Charles’s law and can be represented mathematically as follows:
Gay-Lussac's law shows the relationship between pressure and temperature. Boyle's law shows the relationship between pressure and volume. Newton's third law is not related to gas principles and states that for every force on an object, there is an equal and opposite force of the object on the source of force.
Compare your answer with the correct one above
A balloon filled with room temperature air ( ) has a volume of
) has a volume of  . The balloon is taken outside on a hot summer day where the temperature is
. The balloon is taken outside on a hot summer day where the temperature is  . What will the volume of the balloon be after it is taken outside?
. What will the volume of the balloon be after it is taken outside?
A balloon filled with room temperature air (


We expect the volume to increase since volume and temperature are directly proportional. We know that if we heat something the material will expand so we shouldn't get a value that is smaller than our initial volume. Charles Law says that

where the stuff on the left is the initial volume and temperature and the stuff on the right is the final volume and temperature. First off, we MUST convert the temperatures to Kelvin to use Charles Law. This gives


Solving for the final volume,


We expect the volume to increase since volume and temperature are directly proportional. We know that if we heat something the material will expand so we shouldn't get a value that is smaller than our initial volume. Charles Law says that
where the stuff on the left is the initial volume and temperature and the stuff on the right is the final volume and temperature. First off, we MUST convert the temperatures to Kelvin to use Charles Law. This gives
Solving for the final volume,
Compare your answer with the correct one above
An ideal gas exerts a pressure of  in a
in a  container. The container is at a temperature of
container. The container is at a temperature of  .
.
What will be the final pressure if the volume of the container changes to  ?
?
An ideal gas exerts a pressure of 


What will be the final pressure if the volume of the container changes to 
Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:

Use the given volumes and the initial pressure to solve for the final pressure.


Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:
Use the given volumes and the initial pressure to solve for the final pressure.
Compare your answer with the correct one above
A sample of oxygen gas has a volume of  when its pressure is
when its pressure is  . What will the volume of the gas be at a pressure of
. What will the volume of the gas be at a pressure of  if the temperature remains constant?
if the temperature remains constant?
A sample of oxygen gas has a volume of 


To solve this question we will need to use Boyle's law:

We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.



To solve this question we will need to use Boyle's law:
We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.
Compare your answer with the correct one above
A helium balloon has a volume of  when it is at ground level. The balloon is transported to an elevation of
when it is at ground level. The balloon is transported to an elevation of  , where the pressure is only
, where the pressure is only  . At this altitude the gas occupies a volume of
. At this altitude the gas occupies a volume of  . Assuming the temperature has remained the same, what was the ground level pressure?
. Assuming the temperature has remained the same, what was the ground level pressure?
A helium balloon has a volume of 



To solve this question we will need to use Boyle's law:

We are given the final pressure and volume, along with the initial volume. Using these values, we can calculate the initial pressure.



Note that the pressure at sea level is equal to  . A pressure greater than
. A pressure greater than  simply indicates that the ground level is below sea level at this point.
simply indicates that the ground level is below sea level at this point.
To solve this question we will need to use Boyle's law:
We are given the final pressure and volume, along with the initial volume. Using these values, we can calculate the initial pressure.
Note that the pressure at sea level is equal to 

Compare your answer with the correct one above
What law is the following formula?

What law is the following formula?
Boyle's law relates the pressure and volume of a system, which are inversely proportional to one another. When the parameters of a system change, Boyle's law helps us anticipate the effect the changes have on pressure and volume.

Charles's law relates temperature and volume: 
Gay-Lussac's law relates temperature and pressure: 
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law: 
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant: 
Boyle's law relates the pressure and volume of a system, which are inversely proportional to one another. When the parameters of a system change, Boyle's law helps us anticipate the effect the changes have on pressure and volume.
Charles's law relates temperature and volume:
Gay-Lussac's law relates temperature and pressure:
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law:
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant:
Compare your answer with the correct one above
The graph depicted here represents which of the gas laws?

The graph depicted here represents which of the gas laws?
The graph shows that there is an inverse relationship between the volume and pressure of a gas, when kept at a constant temperature. This was described by Robert Boyle and can be represented mathematically as Boyle's law:

Gay-Lussac's law shows the relationship between pressure and temperature. Charles's law shows the relationship between volume and temperature. Hund's rule (Hund's law) is not related to gases, and states that electron orbitals of an element will be filled with single electrons before any electrons will form pairs within a single orbital.
The graph shows that there is an inverse relationship between the volume and pressure of a gas, when kept at a constant temperature. This was described by Robert Boyle and can be represented mathematically as Boyle's law:
Gay-Lussac's law shows the relationship between pressure and temperature. Charles's law shows the relationship between volume and temperature. Hund's rule (Hund's law) is not related to gases, and states that electron orbitals of an element will be filled with single electrons before any electrons will form pairs within a single orbital.
Compare your answer with the correct one above
A gas is initially in a 5L piston with a pressure of 1atm.
If pressure changes to 3.5atm by moving the piston down, what is new volume?
A gas is initially in a 5L piston with a pressure of 1atm.
If pressure changes to 3.5atm by moving the piston down, what is new volume?
Use Boyle's Law:

Plug in known values and solve for final volume.


Use Boyle's Law:
Plug in known values and solve for final volume.
Compare your answer with the correct one above
A balloon of volume  at
at  is placed in a pressure chamber where the pressure becomes
is placed in a pressure chamber where the pressure becomes  , determine the new volume.
, determine the new volume.
A balloon of volume 


Use Boyle's law and plug in appropriate parameters:




Use Boyle's law and plug in appropriate parameters:
Compare your answer with the correct one above
A gas in a  container is at
container is at  is compressed to a volume of
is compressed to a volume of  . What is the new pressure of the container?
. What is the new pressure of the container?
A gas in a 


Boyle's Law is:

The initial volume ( ) and pressure (
) and pressure ( ) of the gas is given. The volume changes to a new volume (
) of the gas is given. The volume changes to a new volume ( ). Our goal is to find the new pressure (
). Our goal is to find the new pressure ( ). Solving for the new pressure gives:
). Solving for the new pressure gives:


Notice the answer has 3 significant figures.
Boyle's Law is:
The initial volume (



Notice the answer has 3 significant figures.
Compare your answer with the correct one above
Which of the following is a condition of the ideal gas law?
Which of the following is a condition of the ideal gas law?
The ideal gas law has some conditions that must be met, conditions that certainly cannot be met in the real world. These conditions include that the gases cannot interact with one another, gases must be moving in a random straight-line fashion, gas molecules must not take up any space, and gases must be in perfect elastic collisions with the walls of the container. These conditions minimize the effect that gas molecules have on one other, allowing a prediction based on completely random and unimpeded molecular movement. In reality, these conditions are impossible. All real gas molecules have a molecular volume and some degree of intermolecular attraction forces.
The ideal gas law has some conditions that must be met, conditions that certainly cannot be met in the real world. These conditions include that the gases cannot interact with one another, gases must be moving in a random straight-line fashion, gas molecules must not take up any space, and gases must be in perfect elastic collisions with the walls of the container. These conditions minimize the effect that gas molecules have on one other, allowing a prediction based on completely random and unimpeded molecular movement. In reality, these conditions are impossible. All real gas molecules have a molecular volume and some degree of intermolecular attraction forces.
Compare your answer with the correct one above
Which law is represented by the following formula?

Which law is represented by the following formula?
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law.

It is able to relate temperature, pressure, and volume of one system when the parameters for any of the three change.
Boyle's law relates pressure and volume: 
Charles's law relates temperature and volume: 
Gay-Lussac's law relates temperature and pressure: 
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant: 
The combined gas law takes Boyle's, Charles's, and Gay-Lussac's law and combines it into one law.
It is able to relate temperature, pressure, and volume of one system when the parameters for any of the three change.
Boyle's law relates pressure and volume:
Charles's law relates temperature and volume:
Gay-Lussac's law relates temperature and pressure:
The ideal gas law relates temperature, pressure, volume, and moles in coordination with the ideal gas constant:
Compare your answer with the correct one above
A scuba diver uses compressed air to breath under water. He starts with an air volume of  at sea level (
at sea level ( ) at a temperature of
) at a temperature of  . What is the volume of air in his tank at a depth of
. What is the volume of air in his tank at a depth of  (
( ) and a temperature of
) and a temperature of  ?
?
A scuba diver uses compressed air to breath under water. He starts with an air volume of 





This question requires the combined equation of the individual gas laws:

To use this equation, we first need to convert the given temperatures to Kelvin.


We now know the initial pressure, volume, and temperature, allowing us to solve the left side of the equation.


Use the given values for the final temperature and pressure to solve for the final volume.




This question requires the combined equation of the individual gas laws:
To use this equation, we first need to convert the given temperatures to Kelvin.
We now know the initial pressure, volume, and temperature, allowing us to solve the left side of the equation.
Use the given values for the final temperature and pressure to solve for the final volume.
Compare your answer with the correct one above
A  sample of helium gas at
sample of helium gas at  and a pressure of
and a pressure of  is used to inflate a balloon. What is the volume this gas occupies when the temperature reaches
is used to inflate a balloon. What is the volume this gas occupies when the temperature reaches  at a pressure of
at a pressure of  during the inflation?
during the inflation?
A 




This question requires s to use the combined gas law:

We know the initial pressure, temperature, and volume, allowing us to solve for the left side of the equation.


We are also given the final pressure and temperature. Using these values on the right side of the equation we can solve for the final volume.




This question requires s to use the combined gas law:
We know the initial pressure, temperature, and volume, allowing us to solve for the left side of the equation.
We are also given the final pressure and temperature. Using these values on the right side of the equation we can solve for the final volume.
Compare your answer with the correct one above
A  container of gas has a pressure of
container of gas has a pressure of  at a temperature of
at a temperature of  . The container is expanded to
. The container is expanded to  , and the temperature is increased to
, and the temperature is increased to  .
.
What is the final pressure of the container?
A 


, and the temperature is increased to

What is the final pressure of the container?
In this case, two variables are changed between the initial and final containers: volume and temperature. Since we are looking for the final pressure on the container, we can use the combined gas law in order to solve for the final pressure:

When using the ideal gas law, remember that temperature must be in Kelvin, not Celsius, so we will need to convert.


Use the given values to solve for the final pressure.


In this case, two variables are changed between the initial and final containers: volume and temperature. Since we are looking for the final pressure on the container, we can use the combined gas law in order to solve for the final pressure:
When using the ideal gas law, remember that temperature must be in Kelvin, not Celsius, so we will need to convert.
Use the given values to solve for the final pressure.
Compare your answer with the correct one above
 of an unknown gas are contained in a
of an unknown gas are contained in a  container. The container has a pressure of
container. The container has a pressure of  at a temperature of
at a temperature of  .
.
Based on this information and the periodic table, which of the following gases is in the container?





Based on this information and the periodic table, which of the following gases is in the container?
In order to determine the gas being contained, we are going to need to rewrite the ideal gas law. The ideal gas law is written as follows:

The number of moles of a gas can be rewritten as the mass of the gas divided by its molar mass. Knowing this, we can rewrite the equation, and solve for the molar mass of the gas.



Use the given values in this equation to solve for the molar mass. Remember to first convert degree Celsius to Kelvin!


Now that we have solved for the molar mass, we can see which gas has this molar mass on the periodic table. Argon has a molar mass of 39.95 grams per mole, so we can determine that argon is the gas in the container.
In order to determine the gas being contained, we are going to need to rewrite the ideal gas law. The ideal gas law is written as follows:
The number of moles of a gas can be rewritten as the mass of the gas divided by its molar mass. Knowing this, we can rewrite the equation, and solve for the molar mass of the gas.
Use the given values in this equation to solve for the molar mass. Remember to first convert degree Celsius to Kelvin!
Now that we have solved for the molar mass, we can see which gas has this molar mass on the periodic table. Argon has a molar mass of 39.95 grams per mole, so we can determine that argon is the gas in the container.
Compare your answer with the correct one above