Thermodynamics - AP Chemistry
Card 0 of 1235
Which of the following is the correct molar specific heat of water used when making calculations involving a calorimeter?
Which of the following is the correct molar specific heat of water used when making calculations involving a calorimeter?
4.184 J/gK is the cited value for the specific heat of water and should be memorized. This is used during calorimeter calculations, specifically when using the equation q= mc delta(T).
4.184 J/gK is the cited value for the specific heat of water and should be memorized. This is used during calorimeter calculations, specifically when using the equation q= mc delta(T).
Compare your answer with the correct one above
In which instance would a bomb calorimeter be more useful than a coffee-cup calorimeter?
In which instance would a bomb calorimeter be more useful than a coffee-cup calorimeter?
Bomb calorimeters are most useful when dealing with a gas, because they can operate well at high pressures. Coffee-cup calorimeters are not useful when water begins to boil, producing vapor.
Bomb calorimeters are most useful when dealing with a gas, because they can operate well at high pressures. Coffee-cup calorimeters are not useful when water begins to boil, producing vapor.
Compare your answer with the correct one above
How much heat does it take to heat 100g ice at 0C to boiling point?
Cice= 2.1 J/goC
Cwater= 4.2 J/goC
ΔHvap= 2260 J/g
ΔHfus=334 J/g
How much heat does it take to heat 100g ice at 0C to boiling point?
Cice= 2.1 J/goC
Cwater= 4.2 J/goC
ΔHvap= 2260 J/g
ΔHfus=334 J/g
You need heat for the phase change, using the enthalpy of fusion (100g*334 J/g = 33400 J). Add to this the heat to get to boiling point using the specific heat of water (100g*100C*4.2 J/goC = 42000 J). Totalling 75400 J (75.4 kJ)
You need heat for the phase change, using the enthalpy of fusion (100g*334 J/g = 33400 J). Add to this the heat to get to boiling point using the specific heat of water (100g*100C*4.2 J/goC = 42000 J). Totalling 75400 J (75.4 kJ)
Compare your answer with the correct one above
A 50g sample of a metal was heated to  then quickly transferred to an insulated container containing 50g of
then quickly transferred to an insulated container containing 50g of  at
at  . The final temperature of the
. The final temperature of the  was
was  .
.
Which of the following can be concluded?
A 50g sample of a metal was heated to 




Which of the following can be concluded?
When the heated metal is placed in the container of the cooler water there will be a transfer of thermal energy from the metal to the water. This transfer will occur towards an equilibrium of thermal energy in the water and in the metal. Thus we can conclude that the amount of thermal energy lost by the metal will equal the amount of thermal energy gained by the water. However we notice that the water increases by only 5oC and the metal decreases by 65oC. This is becasue of the difference of the specific heats of these substances. The specific heat capacity of a substance is the heat required to increase the temperature of 1g of a substance by 1oC. The metal can be conluded to have a smaller specific heat than the water because the same amount of energy transfer led to a much larger change in termperature for the metal as compared to the water.
When the heated metal is placed in the container of the cooler water there will be a transfer of thermal energy from the metal to the water. This transfer will occur towards an equilibrium of thermal energy in the water and in the metal. Thus we can conclude that the amount of thermal energy lost by the metal will equal the amount of thermal energy gained by the water. However we notice that the water increases by only 5oC and the metal decreases by 65oC. This is becasue of the difference of the specific heats of these substances. The specific heat capacity of a substance is the heat required to increase the temperature of 1g of a substance by 1oC. The metal can be conluded to have a smaller specific heat than the water because the same amount of energy transfer led to a much larger change in termperature for the metal as compared to the water.
Compare your answer with the correct one above
The specific heat capacity of an unknown liquid is 0.32$\frac{J}{kgcdot K}$. The density of the liquid is 0.0321 $\frac{g}{mL}$ If a chemist applies 243 J of heat to 300 mL of this liquid starting at $27.1^{circ}$C, what is the final temperature?
The specific heat capacity of an unknown liquid is 0.32$\frac{J}{kgcdot K}$. The density of the liquid is 0.0321 $\frac{g}{mL}$ If a chemist applies 243 J of heat to 300 mL of this liquid starting at $27.1^{circ}$C, what is the final temperature?
First we will determine the mass of the liquid:
300hspace{1 mm}mLtimes$\frac{0.0321hspace{1 mm}$g}{1hspace{1 mm}mL}=9.63hspace{1 mm}g
Now we will examine the relationship between heat and specific heat capacity:
Q=cmDelta T
Where Q is heat in Joules, c is the specific heat capacity, m is the mass and Delta T is the change in temperature. We can rearrange this
Delta T=\frac{Q}{cm}$
Delta T=\frac{243hspace{1 mm}$J}{0.32Jcdot $kg^{-1}$cdot $K^{-1}$0.00963kg}=78855hspace{1 mm}K
If we begin at $27.1^{circ}$C, we will end at $78882^{circ}$C
First we will determine the mass of the liquid:
300hspace{1 mm}mLtimes$\frac{0.0321hspace{1 mm}$g}{1hspace{1 mm}mL}=9.63hspace{1 mm}g
Now we will examine the relationship between heat and specific heat capacity:
Q=cmDelta T
Where Q is heat in Joules, c is the specific heat capacity, m is the mass and Delta T is the change in temperature. We can rearrange this
Delta T=\frac{Q}{cm}$
Delta T=\frac{243hspace{1 mm}$J}{0.32Jcdot $kg^{-1}$cdot $K^{-1}$0.00963kg}=78855hspace{1 mm}K
If we begin at $27.1^{circ}$C, we will end at $78882^{circ}$C
Compare your answer with the correct one above
The following is a list of specific heat capacities for a few metals.
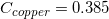
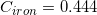
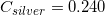

A 50g sample of an unknown metal is heated with 800 joules. If the temperature of the metal increases by 41.6oC, what is the identity of the unknown metal?
The following is a list of specific heat capacities for a few metals.
A 50g sample of an unknown metal is heated with 800 joules. If the temperature of the metal increases by 41.6oC, what is the identity of the unknown metal?
We need to find the specific heat of the unknown sample of metal in order to locate it on the list. We can do this by using the equation that allows us to determine the specific heat capacity of an element.

Since we know the change in temperature, we can simply plug in the values and solve for the value of  .
.


Going back to the list, we see that this is the specific heat capacity for copper, so we confirm that the unknown metal is copper.
We need to find the specific heat of the unknown sample of metal in order to locate it on the list. We can do this by using the equation that allows us to determine the specific heat capacity of an element.
Since we know the change in temperature, we can simply plug in the values and solve for the value of 
Going back to the list, we see that this is the specific heat capacity for copper, so we confirm that the unknown metal is copper.
Compare your answer with the correct one above
A 20g sample of iron at a temperature of  is placed into a container of water. There are 300 milliliters of water in the container at a temperature of
is placed into a container of water. There are 300 milliliters of water in the container at a temperature of  .
.



What is the final temperature of the water?
A 20g sample of iron at a temperature of 

What is the final temperature of the water?
There are two things to note before solving for the final temperature.
1. The density of water allows us to say that 300 milliliters of water is the same thing as 300 grams of water.

2. Since the heat from the iron is being transferred to the water, we can say that the heat transfer is equal between both compounds. Since the heat is conserved in the system, we can set the two equations equal to one another.



Notice how the change in temperature for iron has been flipped in order to avoid a negative number.


Because water has a much higher heat capacity compared to iron, the temperature of the water is not changed significantly.
There are two things to note before solving for the final temperature.
1. The density of water allows us to say that 300 milliliters of water is the same thing as 300 grams of water.
2. Since the heat from the iron is being transferred to the water, we can say that the heat transfer is equal between both compounds. Since the heat is conserved in the system, we can set the two equations equal to one another.
Notice how the change in temperature for iron has been flipped in order to avoid a negative number.
Because water has a much higher heat capacity compared to iron, the temperature of the water is not changed significantly.
Compare your answer with the correct one above



How much energy is needed to raise the temperature of five grams of ice from  to
to  ?
?
How much energy is needed to raise the temperature of five grams of ice from 

This question involves the total energy needed for three different processes: the temperature raise from  to
to  , the melting of the ice, and the temperature raise from
, the melting of the ice, and the temperature raise from  to
to  . For the first and third transitions we will use the equation
. For the first and third transitions we will use the equation  . For the melting of ice, we will use the equation
. For the melting of ice, we will use the equation  .
.
1. 
2. 
3. 
Finally, we will need to sum the energy required for each step to find the total energy.

This question involves the total energy needed for three different processes: the temperature raise from 





1.
2.
3.
Finally, we will need to sum the energy required for each step to find the total energy.
Compare your answer with the correct one above
How many grams of Cr can be obtained by the electrolysis of a Cr(NO3)3 if 10 amps are passed through the cell for 6 hours?
How many grams of Cr can be obtained by the electrolysis of a Cr(NO3)3 if 10 amps are passed through the cell for 6 hours?
Compare your answer with the correct one above
You want to prepare a cup of tea. To do so, you pour  of tap water at
of tap water at  in a cup that does not absorb microwave radiation and heat it in a microwave oven at
in a cup that does not absorb microwave radiation and heat it in a microwave oven at  of power. If you assume a density of
of power. If you assume a density of  for the water and know that its specific heat capacity is
for the water and know that its specific heat capacity is  , what time do you need to set in the microwave oven to heat the water to
, what time do you need to set in the microwave oven to heat the water to  ?
?
You want to prepare a cup of tea. To do so, you pour 






Since the density of water is  , the mass of
, the mass of  is
is  . Plug in known values to the equation and solve.
. Plug in known values to the equation and solve.

Use the formula below to find the time needed to heat up the sample of water in the microwave:


Our answer must contain three significant figures.
Since the density of water is 


Use the formula below to find the time needed to heat up the sample of water in the microwave:
Our answer must contain three significant figures.
Compare your answer with the correct one above
How much heat is needed to raise  grams of aluminum by
grams of aluminum by  ?
?
 .
.
How much heat is needed to raise 


To find the amount of heat needed to change the temperature of a given material by a certain amount, we'll need to use the equation for specific heat. The specific heat capacity of a compound represents the amount of energy necessary to raise  gram of that substance by
gram of that substance by  .
.


To find the amount of heat needed to change the temperature of a given material by a certain amount, we'll need to use the equation for specific heat. The specific heat capacity of a compound represents the amount of energy necessary to raise 

Compare your answer with the correct one above
1. 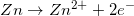

2. 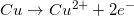

3. 
What is  ? Is reaction 3 spontaneous?
? Is reaction 3 spontaneous?
1. 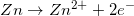
2. 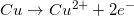
3.
What is 
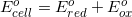
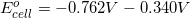

There are two concepts to consider in this problem. First, the question asks for the  . Reaction 3 has
. Reaction 3 has  being reduced so the potential for the half reaction becomes negative.
being reduced so the potential for the half reaction becomes negative.  half reaction appears the same in reaction 3 so the potential is the same. Second, negative voltages indicate non-spontaneity and positive voltages are spontaneous.
half reaction appears the same in reaction 3 so the potential is the same. Second, negative voltages indicate non-spontaneity and positive voltages are spontaneous.
There are two concepts to consider in this problem. First, the question asks for the 


Compare your answer with the correct one above
Consider an electrochemical cell that has the following overall reaction:
2 H+(aq) + Sn (s) -> Sn2+ (aq) + H2 (aq)
Which of the following changes would alter the measured cell potential?
Consider an electrochemical cell that has the following overall reaction:
2 H+(aq) + Sn (s) -> Sn2+ (aq) + H2 (aq)
Which of the following changes would alter the measured cell potential?

All of these changes would change Q and thus change the measured cell potential.
All of these changes would change Q and thus change the measured cell potential.
Compare your answer with the correct one above
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions:
\[Al3+\] = 2.0 M; \[Mn2+\] = 1.0 M.
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions:
\[Al3+\] = 2.0 M; \[Mn2+\] = 1.0 M.

Altering these conditions would increase Q, and thus result in a decrease in the measured cell potential.
Altering these conditions would increase Q, and thus result in a decrease in the measured cell potential.
Compare your answer with the correct one above
Determine the Ecell for the following reaction at 25 C:
Zn (s) + 2 VO2+ (aq) + 4 H+ -> 2 VO2+ (aq) + Zn2+(aq) + 2 H2O (l)
Given that:
VO2+ (aq) + 2 H+ (aq) + e- -> VO2+(aq) + H2O (l) Eo = 1.00 V
Zn2+ (aq) + 2 e--> Zn (s) Eo = -0.76 V
And
\[ VO2+\] = 2.0 M; \[H+\] = 0.50 M; \[VO2+\] = 1.0 x 10-2M; \[Zn2+\] = 1.0 x 10-1M
Determine the Ecell for the following reaction at 25 C:
Zn (s) + 2 VO2+ (aq) + 4 H+ -> 2 VO2+ (aq) + Zn2+(aq) + 2 H2O (l)
Given that:
VO2+ (aq) + 2 H+ (aq) + e- -> VO2+(aq) + H2O (l) Eo = 1.00 V
Zn2+ (aq) + 2 e--> Zn (s) Eo = -0.76 V
And
\[ VO2+\] = 2.0 M; \[H+\] = 0.50 M; \[VO2+\] = 1.0 x 10-2M; \[Zn2+\] = 1.0 x 10-1M
Compare your answer with the correct one above
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions: \[Al3+\] = 1.0 M; \[Mn2+\] = 5.0 M.
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions: \[Al3+\] = 1.0 M; \[Mn2+\] = 5.0 M.

At these concentrations Q will become smaller, and thus log Q will become smaller. This will give rise to a larger cell potential.
At these concentrations Q will become smaller, and thus log Q will become smaller. This will give rise to a larger cell potential.
Compare your answer with the correct one above
What is the cell potential of the following cell:
Zn (s) + 2 H+(aq) -> Zn2+ (aq) + H2 (g) Eo = 0.76 V
When the \[Zn2+\] = 1.0 M; PH2 = 1 atm, and the pH in the cathode is 5.2?
What is the cell potential of the following cell:
Zn (s) + 2 H+(aq) -> Zn2+ (aq) + H2 (g) Eo = 0.76 V
When the \[Zn2+\] = 1.0 M; PH2 = 1 atm, and the pH in the cathode is 5.2?
Compare your answer with the correct one above
Calculate the standard cell potential of the following reaction:
Cd(s) + MnO2 (s) + 4 H+ (aq) + -> Cd2+ (aq) + Mn2+ (aq) + 2 H2O (l)
Given:
MnO2 (s) + 4 H+ (aq) + 2e- -> Mn2+ (aq) + 2 H2O (l) Eo = 1.23 V
Cd2+ (aq) + 2 e- -> Cd (s) Eo = -0.40 V
Calculate the standard cell potential of the following reaction:
Cd(s) + MnO2 (s) + 4 H+ (aq) + -> Cd2+ (aq) + Mn2+ (aq) + 2 H2O (l)
Given:
MnO2 (s) + 4 H+ (aq) + 2e- -> Mn2+ (aq) + 2 H2O (l) Eo = 1.23 V
Cd2+ (aq) + 2 e- -> Cd (s) Eo = -0.40 V
Eocell = Eo cathode - Eoanode
Eocell = 1.23 – (-0.40) = 1.63 V
Eocell = Eo cathode - Eoanode
Eocell = 1.23 – (-0.40) = 1.63 V
Compare your answer with the correct one above
Calculate the standard cell potential of the following reaction:
Zn (s) + Cu2+ (aq) -> Zn2+ (aq) + Cu (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Cu2+(aq)+ 2 e--> Cu (s) Eo = 0.34 V
Calculate the standard cell potential of the following reaction:
Zn (s) + Cu2+ (aq) -> Zn2+ (aq) + Cu (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Cu2+(aq)+ 2 e--> Cu (s) Eo = 0.34 V
Eocell = Eo cathode - Eoanode
Eocell = 0.34 – (-0.76) = 1.10 V
Eocell = Eo cathode - Eoanode
Eocell = 0.34 – (-0.76) = 1.10 V
Compare your answer with the correct one above
Calculate the standard cell potential of the following reaction:
Zn (s) + 2 Ag1+ (aq) -> Zn2+ (aq) + 2 Ag (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Ag1+(aq)+ 1 e--> Ag (s) Eo = 0.80 V
Calculate the standard cell potential of the following reaction:
Zn (s) + 2 Ag1+ (aq) -> Zn2+ (aq) + 2 Ag (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Ag1+(aq)+ 1 e--> Ag (s) Eo = 0.80 V
Eocell = Eo cathode - Eoanode
Eocell = 0.80 – (-0.76) = 1.56 V
Eocell = Eo cathode - Eoanode
Eocell = 0.80 – (-0.76) = 1.56 V
Compare your answer with the correct one above







![= 1.76 V - $\frac{0.0592}{2}$: log : $\frac{[1.0 x $10^{-2}$$ $]^2$ [1.0 x $10^{-1}$ $]}{[2.0]^2$ $[0.5]^4$} = 1.89](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172348/gif.latex)


![[H^+ ] = $10^{-pH}$= $10^{-5.2}$ = 6.31 x $10^{-6}$](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172342/gif.latex)

