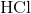Compounds
Help Questions
AP Chemistry › Compounds
Which of the following is the correct name of 
Dinitrogen tetraoxide
Dinitrogen monoxide
Nitrogen monoxide
Nitrogen dioxide
Dinitrogen pentoxide
Explanation
In order to answer the question, it is important to first determine what kind of bonding is occurring in order to properly name the molecule. When nitrogen and oxygen form an 
Because a covalent bond is formed, we can determine that name of the molecule will be based on number of each atom in the molecule. There are two nitrogen atoms in the resulting molecule, so the prefix in front of the "nitrogen" part of the name will be "di-," meaning two. There are four oxygen atoms in the resulting molecule, so the prefix in front of the "oxygen" part of the name will be "tetra," meaning four.
The molecules names will be arranged alphabetically, and the second atom will have "-ide" as the suffix. Therefore, the correct answer is "dinitrogen tetroxide."
What is the oxidation state of manganese (

Explanation
When assigning oxidation states to elements of a given compound, non-transition metal elements are assigned specific oxidation states corresponding to their group number and valence relative to a complete octet.
Group 1 elements have 1 valence electron and an oxidation state of +1.
Group 2 elements have 2 valence electrons and an oxidation state of +2.
Group 8 elements (the noble gases) have a complete octet, thus their assigned oxidation state is 0.
Group 7 elements (halogens) have 7 valence electrons and an oxidation state of -1.
Group 6 elements such as oxygen have 6 valence electrons and an oxidation state of -2.
Permanganate has 4 oxygen atoms and an overall charge of -1. The oxidation state of the 
and finding the difference between their combined oxidation state and the overall charge of the ion (-1):
What is the oxidation state of manganese (

Explanation
When assigning oxidation states to elements of a given compound, non-transition metal elements are assigned specific oxidation states corresponding to their group number and valence relative to a complete octet.
Group 1 elements have 1 valence electron and an oxidation state of +1.
Group 2 elements have 2 valence electrons and an oxidation state of +2.
Group 8 elements (the noble gases) have a complete octet, thus their assigned oxidation state is 0.
Group 7 elements (halogens) have 7 valence electrons and an oxidation state of -1.
Group 6 elements such as oxygen have 6 valence electrons and an oxidation state of -2.
Permanganate has 4 oxygen atoms and an overall charge of -1. The oxidation state of the 
and finding the difference between their combined oxidation state and the overall charge of the ion (-1):
What is the formula for the compound formed from calcium and sulfate ions?
Explanation
In order to answer this question correctly, it is important to have knowledge of the formation of ionic bonds, polyatomic ions, and the periodic table. First, it is important to notice that calcium ions and sulfate ions will form an ionic bond. Knowing this, it can be understood that valance electrons will be exchanged between the ions and therefore, oppositely charged ions that are attracted to each other will be formed.
Calcium is in the second column of the periodic table, so it will provide two ions to the sulfate polyatomic ion. This will form a calcium cation (

One calcium ion provides two electrons, which are both accepted by a sulfate polyatomic ion, so the ratio of calcium ions used to sulfate polyatomic ions used is 1:1. This means that only one of each ion is needed to form the compound, making the formula 
Initially it may be tempting to write the formula as 
All of the following are properties of zinc. Which of the following are chemical properties of zinc?
A. Zinc produces hydrogen gas when placed in acid.
B. Zinc melts at 
C. The density of zinc is 
D. Zinc is a grey metal.
E. Zinc corrodes in moist air.
A and E
C
B
A, C, and E
B and C
Explanation
Physical properties are aspects of matter that can be observed and measured without changing it. Chemical properties measure the potential for undergoing a chemical change. How zinc reacts to acid and in moist air are both chemical properties because the zinc is undergoing chemical change in each environment. The color, density, and melting point of the zinc can all be observed without changing the nature of the matter, so these are physical properties of zinc.
Which of the following are strong electrolytes?
Three
One
Two
Four
All five
Explanation
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Calculate the percent by mass of each element in 
Explanation
The total molar mass of lead (II) sulfate is 



The percent by mass of each element in the compound is found by dividing the mass contribution of that element by the total molar mass of the compound.
Calculate the percent by mass of each element in 
Explanation
The total molar mass of lead (II) sulfate is 



The percent by mass of each element in the compound is found by dividing the mass contribution of that element by the total molar mass of the compound.
What is the percent by mass of bismuth in the compound 
Explanation
The mass percentage of bismuth in the compound will be equal to the mass of bismuth in one mole of compound divided by the total molar mass of the compound.
Bismuth has a molar mass of 
Tellurium has a molar mass of 
Add the mass of bismuth and the mass of tellurium per mole to find the total molar mass.
Divide the mass of bismuth by the total molecular mass to find the percent by mass of bismuth in the compound.
Calculate the percent by mass of each element in 
Explanation
The total mass of one mole of aluminum (II) chromate is calculated by:
Aluminum, chromate, and oxygen contribute