Making Solutions
Help Questions
GED Science › Making Solutions
Questions 1 - 1
1
A student is asked to make 

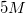
Explanation
Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
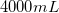
AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Practice TestsTest your skills with exam questions
GamesLearn through free games













