Analytical Chemistry - GRE
Card 0 of 384
Household vinegar contains the organic compound acetic acid with chemical formula,  . If a
. If a  vinegar sample contains
vinegar sample contains  of acetic acid, calculate the percent (mass/mass) of the
of acetic acid, calculate the percent (mass/mass) of the  in the vinegar sample.
in the vinegar sample.
Density of vinegar is the following:

Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Convert the moles of 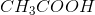 to grams:
to grams:

To calculate the percentage of  in the vinegar we need to use the following formula:
in the vinegar we need to use the following formula:

Therefore,

Convert the moles of 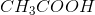
To calculate the percentage of 
Therefore,
Compare your answer with the correct one above
An alcohol group in a compound would result in a broad dip around what part of the infrared (IR) spectrum?
An alcohol group in a compound would result in a broad dip around what part of the infrared (IR) spectrum?
There are a couple of key functional group spectra that you must memorize. A carbonyl group will cause a sharp dip at about 1700cm-1, and an alcohol group will cause a broad dip around 3400cm-1.
There are a couple of key functional group spectra that you must memorize. A carbonyl group will cause a sharp dip at about 1700cm-1, and an alcohol group will cause a broad dip around 3400cm-1.
Compare your answer with the correct one above
An unknown compound is analyzed using infrared spectroscopy. A strong, sharp peak is observed at a frequency of 1750cm-1. What functional group is present?
An unknown compound is analyzed using infrared spectroscopy. A strong, sharp peak is observed at a frequency of 1750cm-1. What functional group is present?
An ester has a characteristic IR absorption at about 1750cm-1. A saturated ketone has an absorption at about 1710cm-1, while an unsaturated ketone has an absorption between 1650cm-1 and 1700cm-1. A nitrile has an IR frequency of about 2200cm-1, while an alcohol has a strong, broad peak at about 3400cm-1.
Carbonyl compounds all have peaks between roughly 1650cm-1 and 1750cm-1. Ketone peaks are generally observed at the lower end of this range, while aldehydes and esters are toward the higher end of the range.
An ester has a characteristic IR absorption at about 1750cm-1. A saturated ketone has an absorption at about 1710cm-1, while an unsaturated ketone has an absorption between 1650cm-1 and 1700cm-1. A nitrile has an IR frequency of about 2200cm-1, while an alcohol has a strong, broad peak at about 3400cm-1.
Carbonyl compounds all have peaks between roughly 1650cm-1 and 1750cm-1. Ketone peaks are generally observed at the lower end of this range, while aldehydes and esters are toward the higher end of the range.
Compare your answer with the correct one above
In IR spectroscopy, the vibration between atoms is caused by which of the following?
In IR spectroscopy, the vibration between atoms is caused by which of the following?
Infrared (IR) spectroscopy takes advantage of the electrical difference between atoms in a polar bond. These dipole moments, when exposed to infrared radiation, stretch and contract in what appears to be a vibrating motion between the atoms. The different vibrational frequencies in the molecule allow for the compound to be "read" using IR spectroscopy.
Infrared (IR) spectroscopy takes advantage of the electrical difference between atoms in a polar bond. These dipole moments, when exposed to infrared radiation, stretch and contract in what appears to be a vibrating motion between the atoms. The different vibrational frequencies in the molecule allow for the compound to be "read" using IR spectroscopy.
Compare your answer with the correct one above
An IR spectrum reading is taken before and after treating acetone with the reducing agent  . What IR peak readings would be seen for the reactant acetone and for the predicted product?
. What IR peak readings would be seen for the reactant acetone and for the predicted product?
An IR spectrum reading is taken before and after treating acetone with the reducing agent 
Treating acetone, a secondary carbonyl, with a reducing agent, such as sodium borohydride (NaBH4), will yield a secondary alcohol as the product.
When using IR spectroscopy, carbonyl (C=O) groups display characteristic peaks at approximately 1700cm-1, while alcohol groups (O-H) display characteristic peaks around 3300cm-1. The acetone would, therefore, initially have a characteristic peak at roughly 1700cm-1. After the reduction reaction is complete, the resulting 2-propanol would display a characteristic peak roughly at 3300cm-1.
Treating acetone, a secondary carbonyl, with a reducing agent, such as sodium borohydride (NaBH4), will yield a secondary alcohol as the product.
When using IR spectroscopy, carbonyl (C=O) groups display characteristic peaks at approximately 1700cm-1, while alcohol groups (O-H) display characteristic peaks around 3300cm-1. The acetone would, therefore, initially have a characteristic peak at roughly 1700cm-1. After the reduction reaction is complete, the resulting 2-propanol would display a characteristic peak roughly at 3300cm-1.
Compare your answer with the correct one above
Approximately where would a carbonyl peak be found on an IR spectrum?
Approximately where would a carbonyl peak be found on an IR spectrum?
It is important to memorize a couple key functional groups, and where they are located on an IR spectrum. If you see a sharp peak near 1700cm-1, you can assume it is made by a carbonyl group.
Similarly, a wide peak around 3000cm-1 will be made by a hydroxyl group.
It is important to memorize a couple key functional groups, and where they are located on an IR spectrum. If you see a sharp peak near 1700cm-1, you can assume it is made by a carbonyl group.
Similarly, a wide peak around 3000cm-1 will be made by a hydroxyl group.
Compare your answer with the correct one above
Which of the following statements is true concerning infrared spectroscopy?
Which of the following statements is true concerning infrared spectroscopy?
IR spectroscopy allows you to identify what functional groups are present in a compound. The IR spectrum is created by recording the frequencies at which a polar bond's vibration frequency is equal to the infrared light's frequency.
The fingerprint region is separate from the function group region, and generally corresponds to carbon-carbon or carbon-hydrogen interactions. While the spectrum can show what groups are present in a compound, it cannot be used to find the position of these groups or provide a carbon skeleton.
IR spectroscopy allows you to identify what functional groups are present in a compound. The IR spectrum is created by recording the frequencies at which a polar bond's vibration frequency is equal to the infrared light's frequency.
The fingerprint region is separate from the function group region, and generally corresponds to carbon-carbon or carbon-hydrogen interactions. While the spectrum can show what groups are present in a compound, it cannot be used to find the position of these groups or provide a carbon skeleton.
Compare your answer with the correct one above
Which of the following functional groups exhibits the highest frequency in an infrared (IR) spectrum?
Which of the following functional groups exhibits the highest frequency in an infrared (IR) spectrum?
An alcohol (-ROH) exhibits a strong, broad absorbance peak at about 3500cm-1. A nitrile's (-RCN) characteristic absorbance peak is at about 2200cm-1. Carbonyl groups have strong, sharp peaks from 1700cm-1 to 1750cm-1, depending on the type of carbonyl group. For instance, an ester (-RCO2R'-) has an absorbance at about 1750cm-1, while a ketone (-ROR'-) has an absorbance at around 1710cm-1.
An alcohol (-ROH) exhibits a strong, broad absorbance peak at about 3500cm-1. A nitrile's (-RCN) characteristic absorbance peak is at about 2200cm-1. Carbonyl groups have strong, sharp peaks from 1700cm-1 to 1750cm-1, depending on the type of carbonyl group. For instance, an ester (-RCO2R'-) has an absorbance at about 1750cm-1, while a ketone (-ROR'-) has an absorbance at around 1710cm-1.
Compare your answer with the correct one above
Which of the following statements is true concerning infrared (IR) spectroscopy?
Which of the following statements is true concerning infrared (IR) spectroscopy?
IR spectroscopy is most commonly used to determine the functional groups found in the molecule being observed. This is done by observing the vibration frequencies between atoms in the molecule. It does not easily reveal the size or shape of the molecule's carbon skeleton. Although the fingerprint region is unique for every molecule, it is very difficult to read when attempting to determine the molecule's functional groups. Most functional group peaks are observed in the functional group region adjacent to the fingerprint region.
IR spectroscopy is most commonly used to determine the functional groups found in the molecule being observed. This is done by observing the vibration frequencies between atoms in the molecule. It does not easily reveal the size or shape of the molecule's carbon skeleton. Although the fingerprint region is unique for every molecule, it is very difficult to read when attempting to determine the molecule's functional groups. Most functional group peaks are observed in the functional group region adjacent to the fingerprint region.
Compare your answer with the correct one above
After taking an IR spectrum of a sample synthesized in the lab, you have 3 IR peaks. Peak  has a
has a  transmittance, peak
transmittance, peak  has a
has a  transmittance, and peak
transmittance, and peak  has a
has a  transmittance. Which peak has the greatest absorbance?
transmittance. Which peak has the greatest absorbance?
After taking an IR spectrum of a sample synthesized in the lab, you have 3 IR peaks. Peak 





Transmittance ( ) is the fraction of incident light transmitted through an analyte. Absorbance (
) is the fraction of incident light transmitted through an analyte. Absorbance ( ) is the amount incident light that is absorbed by the analyte. The equation that governs this relationship is:
) is the amount incident light that is absorbed by the analyte. The equation that governs this relationship is:

Where  is the power of the incident radiation and
is the power of the incident radiation and  is the decreased power of the incident radiation due to the interactions between the absorbing analyte particles and the power of the incident radiation. So, as the percent transmittance increases the absorbance decreases. In this case, peak
is the decreased power of the incident radiation due to the interactions between the absorbing analyte particles and the power of the incident radiation. So, as the percent transmittance increases the absorbance decreases. In this case, peak  has the lowest transmittance, therefore it has the highest absorbance.
has the lowest transmittance, therefore it has the highest absorbance.
Transmittance (

Where 


Compare your answer with the correct one above
What is the absorbance of an IR peak with a 25% transmittance?
What is the absorbance of an IR peak with a 25% transmittance?
There are two equations we can use to solve this question:

And

Let's show that each give us the same correct answer:


There are two equations we can use to solve this question:
And
Let's show that each give us the same correct answer:
Compare your answer with the correct one above
What is the absorbance of an IR peak with a  transmittance?
transmittance?
What is the absorbance of an IR peak with a 

Therefore,
Using the equation that relates absorbance to transmittance, we can convert 0.17 transmittance to absorbance:

Therefore the absorbance for this peak is 0.77.
Therefore,
Using the equation that relates absorbance to transmittance, we can convert 0.17 transmittance to absorbance:
Therefore the absorbance for this peak is 0.77.
Compare your answer with the correct one above
What is the absorbance of an IR peak with a  transmittance?
transmittance?
What is the absorbance of an IR peak with a 

Therefore,
Using the equation that relates absorbance to transmittance, we can convert 0.36 transmittance to absorbance:

Therefore the absorbance for this peak is 0.44.
Therefore,
Using the equation that relates absorbance to transmittance, we can convert 0.36 transmittance to absorbance:
Therefore the absorbance for this peak is 0.44.
Compare your answer with the correct one above
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.


Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).


Use this equation and the absorbance given in the question to find the concentration of the unknown sample.


For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.
Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).
Use this equation and the absorbance given in the question to find the concentration of the unknown sample.
Compare your answer with the correct one above
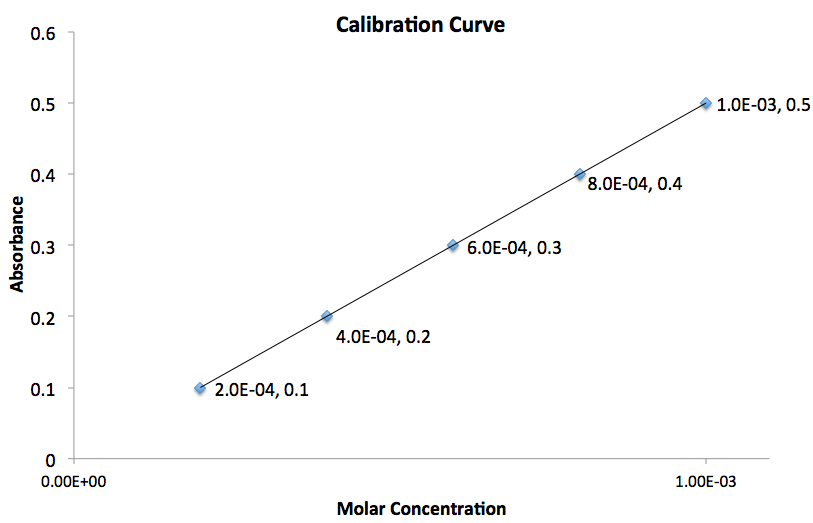
Using the graph given, what is the value for the slope of the line?

Using the graph given, what is the value for the slope of the line?
The slope of the line,  , can be calculated by the following equation:
, can be calculated by the following equation:

The subscripts are simply the points you chose in the order you arbitrarily chose.The most important thing to do is plug the values into equation in the order you chose them.
To determine the slope of the line we need to use two points on the graph. Assuming the two points chosen correspond to  and
and  , plugging these values into the equation gives:
, plugging these values into the equation gives:

Therefore the slope of the line is equal to 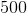
The slope of the line, 
The subscripts are simply the points you chose in the order you arbitrarily chose.The most important thing to do is plug the values into equation in the order you chose them.
To determine the slope of the line we need to use two points on the graph. Assuming the two points chosen correspond to 

Therefore the slope of the line is equal to
Compare your answer with the correct one above
DNA is negatively charged, so it will migrate toward the positive electrode during electrophoresis. As a result, it will migrate from the center line into the green region of the gel.
DNA is negatively charged, so it will migrate toward the positive electrode during electrophoresis. As a result, it will migrate from the center line into the green region of the gel.
Compare your answer with the correct one above
Which of the following would be considered a Lewis base?
Which of the following would be considered a Lewis base?
A lewis base is an electron pair donor.  would be considered a lewis base based on the definition. Because
would be considered a lewis base based on the definition. Because  is electron rich based on its oxidation number, it is able to donate an electron pair to an electron deficient ion.
is electron rich based on its oxidation number, it is able to donate an electron pair to an electron deficient ion.
A lewis base is an electron pair donor. 

Compare your answer with the correct one above
Which molecule would be considered a Lewis acid?
Which molecule would be considered a Lewis acid?
A lewis acid is an electron pair acceptor.  would be considered a lewis acid based on the definition. Because
would be considered a lewis acid based on the definition. Because  is electron-deficient based on its oxidation number, it is able to accept an electron pair.
is electron-deficient based on its oxidation number, it is able to accept an electron pair.
A lewis acid is an electron pair acceptor. 

Compare your answer with the correct one above
Which of the following types of reactions best describes the following reaction:

Which of the following types of reactions best describes the following reaction:
A redox reaction is also known as an oxidation/reduction reaction. This type of reaction involves the transfer of electrons from on reactant to another. In this reaction, an electron is transferred from  to
to  forming the products
forming the products  and
and  . The substance that gains an electron is referred to as being reduced. The substance that loses an electron is referred to as being oxidized.
. The substance that gains an electron is referred to as being reduced. The substance that loses an electron is referred to as being oxidized.
A redox reaction is also known as an oxidation/reduction reaction. This type of reaction involves the transfer of electrons from on reactant to another. In this reaction, an electron is transferred from 



Compare your answer with the correct one above
What is the oxidation state of manganese in  ?
?
What is the oxidation state of manganese in 
In this compound oxygen has an oxidation number of  . There are 4 oxygens, therefore the total oxidation number for the oxygens in
. There are 4 oxygens, therefore the total oxidation number for the oxygens in  is:
is:  . Potassium,
. Potassium, , is a group 1 metal and has an oxidation number of
, is a group 1 metal and has an oxidation number of  . The sum of all oxidation number in a neutral compound such as
. The sum of all oxidation number in a neutral compound such as  is zero. We will give the oxidation number of
is zero. We will give the oxidation number of  equal to
equal to  :
:

Simplifying the above equation:


Rearranging to solve for  :
:

In this compound oxygen has an oxidation number of 







Simplifying the above equation:
Rearranging to solve for 
Compare your answer with the correct one above
