General Chemistry - GRE
Card 0 of 672
What is the molecular shape of  ?
?
What is the molecular shape of 
NH3 is trigonal pyramidal because it has 4 electron domains, one of them being a lone pair of electrons and the other three being H atoms. When this is arranged in a three-dimensional space, it is trigonal pyramidal in shape.
NH3 is trigonal pyramidal because it has 4 electron domains, one of them being a lone pair of electrons and the other three being H atoms. When this is arranged in a three-dimensional space, it is trigonal pyramidal in shape.
Compare your answer with the correct one above
Which of the following is not the correct geometric configuration for the given molecule?
Which of the following is not the correct geometric configuration for the given molecule?
Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Compare your answer with the correct one above
Which of the following molecules exhibits a trigonal pyramidal geometry?
Which of the following molecules exhibits a trigonal pyramidal geometry?
The trigonal pyramidal geometry is implemented by molecules in which the central atom has three atoms and a lone pair attached.  has three hydrogens attached to the central phosphorus as well as a lone pair, which can be determined by drawing the Lewis structure of the molecule. As a result, it has trigonal pyramidal geometry.
has three hydrogens attached to the central phosphorus as well as a lone pair, which can be determined by drawing the Lewis structure of the molecule. As a result, it has trigonal pyramidal geometry.
 is trigonal planar.
is trigonal planar.  is tetrahedral.
is tetrahedral.  is octahedral.
is octahedral.
The trigonal pyramidal geometry is implemented by molecules in which the central atom has three atoms and a lone pair attached. 



Compare your answer with the correct one above
Which of the following molecules has an electronic geometry that is the same as its molecular geometry?
Which of the following molecules has an electronic geometry that is the same as its molecular geometry?
Electronic and molecular geometries are only the same when there are no lone pairs around the central atom in the molecule.  is the only given option that does not have a lone pair on the central atom, so the electronic geometry is the same as the molecular geometry (in this case, tetrahedral).
is the only given option that does not have a lone pair on the central atom, so the electronic geometry is the same as the molecular geometry (in this case, tetrahedral).
Electronic and molecular geometries are only the same when there are no lone pairs around the central atom in the molecule. 
Compare your answer with the correct one above
What is the definition of a Brønsted-Lowry base?
What is the definition of a Brønsted-Lowry base?
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
Compare your answer with the correct one above
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.

All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of  , the proton is acting as a(n) , and is thus a .
, the proton is acting as a(n) , and is thus a .
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.
The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of 

In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the  signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from
signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from  , and is thus acting as a Lewis acid.
, and is thus acting as a Lewis acid.
In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the 

Compare your answer with the correct one above
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore  and
and  are not lewis acids.
are not lewis acids.  is very stable and insoluble and cannot accept an electron pair.
is very stable and insoluble and cannot accept an electron pair.  is a well known base and has extremely weak acidity.
is a well known base and has extremely weak acidity.  is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore 




Compare your answer with the correct one above
Which of the following is not a strong electrophile?
Which of the following is not a strong electrophile?
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge.  is the only option given that contains a negative charge and therefore is not an electrophile.
is the only option given that contains a negative charge and therefore is not an electrophile.
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge. 
Compare your answer with the correct one above
Which of the following molecules will have the largest bond angle?
Which of the following molecules will have the largest bond angle?
According to VSEPR theory, atoms will orient themselves in order to be as far away from neighboring atoms as possible in a molecule. This theory helps predict the geometry that molecules will take, as well as the bond angles between atoms.
 is formed by a central carbon bound to two adjacent oxygen atoms. To maximize the distance between the oxygen atoms, they will align at an angle of 180 degrees, creating a linear shape.
is formed by a central carbon bound to two adjacent oxygen atoms. To maximize the distance between the oxygen atoms, they will align at an angle of 180 degrees, creating a linear shape.
All of the other given moleules will have bond angles less than 180 degrees.
According to VSEPR theory, atoms will orient themselves in order to be as far away from neighboring atoms as possible in a molecule. This theory helps predict the geometry that molecules will take, as well as the bond angles between atoms.

All of the other given moleules will have bond angles less than 180 degrees.
Compare your answer with the correct one above
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.

All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of  , the proton is acting as a(n) , and is thus a .
, the proton is acting as a(n) , and is thus a .
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.
The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of 

In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the  signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from
signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from  , and is thus acting as a Lewis acid.
, and is thus acting as a Lewis acid.
In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the 

Compare your answer with the correct one above
What is the definition of a Brønsted-Lowry base?
What is the definition of a Brønsted-Lowry base?
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
Compare your answer with the correct one above
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore  and
and  are not lewis acids.
are not lewis acids.  is very stable and insoluble and cannot accept an electron pair.
is very stable and insoluble and cannot accept an electron pair.  is a well known base and has extremely weak acidity.
is a well known base and has extremely weak acidity.  is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore 




Compare your answer with the correct one above
Which of the following is not a strong electrophile?
Which of the following is not a strong electrophile?
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge.  is the only option given that contains a negative charge and therefore is not an electrophile.
is the only option given that contains a negative charge and therefore is not an electrophile.
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge. 
Compare your answer with the correct one above
Which of the following is true regarding the solubility product constant,  , for a reaction in the form:
, for a reaction in the form:

Which of the following is true regarding the solubility product constant, 
To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of  . The equation for the solubility product constant of this reaction is:
. The equation for the solubility product constant of this reaction is:
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100575/gif.latex)
The units of the solubility product constant will depend on the coefficients of the products. 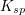 will be a constant for the reaction, and will not change as more solid dissolves or as the reaction progresses.
will be a constant for the reaction, and will not change as more solid dissolves or as the reaction progresses.
To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of 
The units of the solubility product constant will depend on the coefficients of the products. 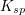
Compare your answer with the correct one above
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.

All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of  , the proton is acting as a(n) , and is thus a .
, the proton is acting as a(n) , and is thus a .
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.
The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
In the reverse reaction of 

In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the  signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from
signifies that it is a Lewis base with available electrons to donate. The proton is accepting these electrons from  , and is thus acting as a Lewis acid.
, and is thus acting as a Lewis acid.
In terms of the passage, the lone proton can be considered a proton donor and would, therefore, be a Brønsted-Lowry acid. This is not an answer choice.
The third acid-base definition is the Lewis definition, which states that acids are electron acceptors and bases are electron donors. The negative charge on the 

Compare your answer with the correct one above
What is the definition of a Brønsted-Lowry base?
What is the definition of a Brønsted-Lowry base?
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
A Brønsted-Lowry base is any compound that accepts protons in solution. Lewis acids and bases refer to the accepting or donating of an electron pair, respectively.
Compare your answer with the correct one above
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Which of the following molecules or ions have the greatest ability to act like a Lewis acid?
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore  and
and  are not lewis acids.
are not lewis acids.  is very stable and insoluble and cannot accept an electron pair.
is very stable and insoluble and cannot accept an electron pair.  is a well known base and has extremely weak acidity.
is a well known base and has extremely weak acidity.  is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
is a transition metal ion. Transition metal are known to be Lewis acids because of their positive charge which gives them the ability to accept electron pairs.
Lewis acids are electron pair acceptors. Molecules and ions that have a full octet cannot act a Lewis acid, therefore 




Compare your answer with the correct one above
Which of the following is not a strong electrophile?
Which of the following is not a strong electrophile?
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge.  is the only option given that contains a negative charge and therefore is not an electrophile.
is the only option given that contains a negative charge and therefore is not an electrophile.
One of the easiest ways of determining if a molecule is an electrophile is by the presence of a positive charge. Electrophiles are in need of electrons, therefore they are electron deficient and can be attacked by nucleophiles (compounds that are electron rich). A nucleophile is a compound that provides a pair of electrons to form a new covalent bond. Nucleophiles are electron rich and one of the easiest types of nucleophiles to recognize are ones carrying a negative charge. 
Compare your answer with the correct one above
What happens when a solution of  is added to a solution of
is added to a solution of  ?
?
What happens when a solution of 

The chemical reaction that would occur is provided below:

This type of reaction is called a double displacement reaction in which there is an exchange of ions between two compounds. This type of reaction usually results in the formation of a precipitate or gas. In terms of the positive ions, the  switch places
switch places  to form two new products.
to form two new products.
The chemical reaction that would occur is provided below:
This type of reaction is called a double displacement reaction in which there is an exchange of ions between two compounds. This type of reaction usually results in the formation of a precipitate or gas. In terms of the positive ions, the 

Compare your answer with the correct one above
Which of the following types of reactions best describes the following reaction:

Which of the following types of reactions best describes the following reaction:
The type of reaction given is termed a combustion reaction. A combustion reaction is one that involves a substance reacting with molecular oxygen. For combustion reactions involving a hydrocarbon as given in the example, the major products are carbon dioxide and water. The word combustion is a synonym for burn.
The type of reaction given is termed a combustion reaction. A combustion reaction is one that involves a substance reacting with molecular oxygen. For combustion reactions involving a hydrocarbon as given in the example, the major products are carbon dioxide and water. The word combustion is a synonym for burn.
Compare your answer with the correct one above