Organic Chemistry, Biochemistry, and Metabolism - MCAT Biological and Biochemical Foundations of Living Systems
Card 0 of 2928
Which of the following is NOT a class of enzyme?
Which of the following is NOT a class of enzyme?
The correct answer is pyrimidine complex. A pyrimidine refers to a type of nucleotide base. Enzymes commonly have the suffix -ase at the end of their name.
The correct answer is pyrimidine complex. A pyrimidine refers to a type of nucleotide base. Enzymes commonly have the suffix -ase at the end of their name.
Compare your answer with the correct one above
Which of the following functional groups would most likely act as an acid?
Which of the following functional groups would most likely act as an acid?
Carboxyl groups, or carboxylic acids, are good acids due to the resonance between the two oxygen atoms, allowing for greater stability of the conjugate base upon removal of a proton. Acetals and aldehydes can act as weak acids, but carboxyl groups will be deprotonated first.
Carboxyl groups, or carboxylic acids, are good acids due to the resonance between the two oxygen atoms, allowing for greater stability of the conjugate base upon removal of a proton. Acetals and aldehydes can act as weak acids, but carboxyl groups will be deprotonated first.
Compare your answer with the correct one above
Which of the following steps of free radical chlorination does not produce a free radical as a product?
Which of the following steps of free radical chlorination does not produce a free radical as a product?
The three steps of a free radical chlorination reaction are, in order, initiation, propagation, and termination.
Free radicals are produced in the initiation and propagation steps. The termination steps combine any two free radicals formed in the reaction to produce a compound that has no unpaired electrons (free radicals).
The three steps of a free radical chlorination reaction are, in order, initiation, propagation, and termination.
Free radicals are produced in the initiation and propagation steps. The termination steps combine any two free radicals formed in the reaction to produce a compound that has no unpaired electrons (free radicals).
Compare your answer with the correct one above
Which of the following amino acids is basic?
Which of the following amino acids is basic?
On the MCAT you must be able to recognize the following as basic amino acids: lysine, arginine, and histidine. Important acidic amino acids include aspartic acid (aspartate) and glutamic acid (glutamate). Important nonpolar amino acids include: methionine, alanine, isoleucine, proline, phenylalanine, tryptophan, valine, and leucine.
On the MCAT you must be able to recognize the following as basic amino acids: lysine, arginine, and histidine. Important acidic amino acids include aspartic acid (aspartate) and glutamic acid (glutamate). Important nonpolar amino acids include: methionine, alanine, isoleucine, proline, phenylalanine, tryptophan, valine, and leucine.
Compare your answer with the correct one above
A depletion of amino acids in a cell would slow which immediate process?
A depletion of amino acids in a cell would slow which immediate process?
A depletion of amino acids would immediately impact protein and enzyme production in the cell. All proteins, including enzymes, are made from chains of amino acids. Lack of nutrients, such as amino acids, would eventually impact other processes listed.
A depletion of amino acids would immediately impact protein and enzyme production in the cell. All proteins, including enzymes, are made from chains of amino acids. Lack of nutrients, such as amino acids, would eventually impact other processes listed.
Compare your answer with the correct one above
Which two functional groups are included in every amino acid, and take part in amino acids binding together?
Which two functional groups are included in every amino acid, and take part in amino acids binding together?
Every amino acid contains a carboxyl group and an amino group. These two functional groups are essential for amino acid binding and breaking.
While sulfide groups contribute to higher protein structure by forming disulfide bonds, they do not exist in every amino acid.
Every amino acid contains a carboxyl group and an amino group. These two functional groups are essential for amino acid binding and breaking.
While sulfide groups contribute to higher protein structure by forming disulfide bonds, they do not exist in every amino acid.
Compare your answer with the correct one above
What are the four categories of amino acids?
What are the four categories of amino acids?
Amino acids are categorized as nonpolar, polar, acidic, or basic. The category that an amino acid is placed into gives you an idea of where you might find the amino acid within a protein. For example, polar amino acids are commonly found on the outside of proteins, where other polar molecules (water) are likely to be found.
Amino acids are categorized as nonpolar, polar, acidic, or basic. The category that an amino acid is placed into gives you an idea of where you might find the amino acid within a protein. For example, polar amino acids are commonly found on the outside of proteins, where other polar molecules (water) are likely to be found.
Compare your answer with the correct one above
During cellular respiration, where is NADH produced?
During cellular respiration, where is NADH produced?
NADH is produced during glycolysis, which occurs in the cytoplasm. NADH is also produced during the Krebs cycle, which occurs in the mitochondrial matrix. The protons generated in the production of NADH are later used in the intermembrane space to power ATP synthase during oxidative phosphorylation.
NADH is produced during glycolysis, which occurs in the cytoplasm. NADH is also produced during the Krebs cycle, which occurs in the mitochondrial matrix. The protons generated in the production of NADH are later used in the intermembrane space to power ATP synthase during oxidative phosphorylation.
Compare your answer with the correct one above
In the reaction scheme below, compound A is a(n) and compound B is a(n) .

In the reaction scheme below, compound A is a(n) and compound B is a(n) .

Ketones, like compound A, contain an internal carbon-oxygen double bond. Alcohols, like compound B, contain a hydroxyl group (-OH). In this case, compound A is a secondary ketone and compound B is a tertiary alcohol.
Alkenes, like compound C, contain a carbon-carbon double bond.
Ketones, like compound A, contain an internal carbon-oxygen double bond. Alcohols, like compound B, contain a hydroxyl group (-OH). In this case, compound A is a secondary ketone and compound B is a tertiary alcohol.
Alkenes, like compound C, contain a carbon-carbon double bond.
Compare your answer with the correct one above
Which compound has the largest bond energy between carbons?
Which compound has the largest bond energy between carbons?
It helps to remember that bond energy is inversely proportional to bond length. In other words, the shorter the bond, the higher the bond energy.
Bond length is shortened when pi bonds are involved, so double and triple bonds are much shorter than simple sigma bonds. The bond length between the carbons in ethyne is the shortest out of all options because they are triple bonded to one another. This also makes it the most stable bond, and gives it the largest bond energy.
Benzene and 1,3-butadiene will have relatively high energy stored in double bonds, but will be unable to match a triple bond. Propane has only single bonds and will have the lowest bond energy of these answers.
It helps to remember that bond energy is inversely proportional to bond length. In other words, the shorter the bond, the higher the bond energy.
Bond length is shortened when pi bonds are involved, so double and triple bonds are much shorter than simple sigma bonds. The bond length between the carbons in ethyne is the shortest out of all options because they are triple bonded to one another. This also makes it the most stable bond, and gives it the largest bond energy.
Benzene and 1,3-butadiene will have relatively high energy stored in double bonds, but will be unable to match a triple bond. Propane has only single bonds and will have the lowest bond energy of these answers.
Compare your answer with the correct one above
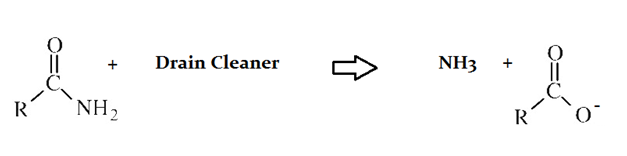
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The C–N bond in the original protein, before reaction with drain cleaner is .
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The C–N bond in the original protein, before reaction with drain cleaner is .
The C–N bond is a single bond, and the carbonyl bond is a double bond. Double bonds are stronger and shorter than single bonds.
The C–N bond is a single bond, and the carbonyl bond is a double bond. Double bonds are stronger and shorter than single bonds.
Compare your answer with the correct one above
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the carbonyl bonds of the preceeding passage .
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the carbonyl bonds of the preceeding passage .
The carbon at the center of a carbonyl group bonds with three sigma bonds, and one pi bond. The pi bond exists above and below the plane of the sigma bond. This carbon thus shows sp2 hybridization.
The carbon at the center of a carbonyl group bonds with three sigma bonds, and one pi bond. The pi bond exists above and below the plane of the sigma bond. This carbon thus shows sp2 hybridization.
Compare your answer with the correct one above
Which organelle would have the most negative effect if its membrane were damaged?
Which organelle would have the most negative effect if its membrane were damaged?
The lysosomes contain an acidic environment and digestive enzymes. Damage to the membrane would allow hydrogen ions and these enzymes to escape into the cytoplasm of the cell, where they would do damage to the other cellular components.
Damage to a mitochondrion or chloroplast would affect energy production in the cell, but would not actively cause damage. Ribosomes don't have membranes.
The lysosomes contain an acidic environment and digestive enzymes. Damage to the membrane would allow hydrogen ions and these enzymes to escape into the cytoplasm of the cell, where they would do damage to the other cellular components.
Damage to a mitochondrion or chloroplast would affect energy production in the cell, but would not actively cause damage. Ribosomes don't have membranes.
Compare your answer with the correct one above
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
What is the predicted molecular orbital hybridization state of the carbon in carbonic acid?
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
What is the predicted molecular orbital hybridization state of the carbon in carbonic acid?
Carbons bound via one double bond are sp2 hybridized, as long as the remaining two bonds are each sigma bonds. Take the number of sigma bonds and subtract one for your exponent in the spx expression.
Carbons bound via one double bond are sp2 hybridized, as long as the remaining two bonds are each sigma bonds. Take the number of sigma bonds and subtract one for your exponent in the spx expression.
Compare your answer with the correct one above
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The bond that is present between the carbon atom and the carbonyl oxygen atom in carbonc acid is best described as having which of the following?
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The bond that is present between the carbon atom and the carbonyl oxygen atom in carbonc acid is best described as having which of the following?
The bond in question is a double bond, and thus is composed of one sigma and one pi bond.
The bond in question is a double bond, and thus is composed of one sigma and one pi bond.
Compare your answer with the correct one above
Under normal circumstances, which of the following carbons will be sp3 hybridized?
Under normal circumstances, which of the following carbons will be sp3 hybridized?
Carbanions are usually sp3 hybridized. Free radical carbons, carbocations, and double bonded carbons are all sp2 hybridized.
Carbanions are usually sp3 hybridized. Free radical carbons, carbocations, and double bonded carbons are all sp2 hybridized.
Compare your answer with the correct one above
When formic acid is completely reduced, methanol is formed.
What is the hybridization of the carbon in formic acid, compared to the carbon in methanol?
When formic acid is completely reduced, methanol is formed.
What is the hybridization of the carbon in formic acid, compared to the carbon in methanol?
In order to find the hybridization of an atom, simply count the number of sigma bonds and lone pair electrons around the atom. The carbon in formic acid is double bonded to an oxygen, and has two single bonds. This means that it has sp2 hybridization. Upon being reduced to methanol, the carbon now has four single bonds surrounding it. As a result, the carbon now has sp3 hybridization.
Remember that a triple bond corresponds to sp hydrization, a double bond to sp2, and single bonds to sp3 for a carbon atom.
In order to find the hybridization of an atom, simply count the number of sigma bonds and lone pair electrons around the atom. The carbon in formic acid is double bonded to an oxygen, and has two single bonds. This means that it has sp2 hybridization. Upon being reduced to methanol, the carbon now has four single bonds surrounding it. As a result, the carbon now has sp3 hybridization.
Remember that a triple bond corresponds to sp hydrization, a double bond to sp2, and single bonds to sp3 for a carbon atom.
Compare your answer with the correct one above
Which compound will have the highest bond energy?
Which compound will have the highest bond energy?
In organic chemistry, the trend is that bond length is inversely proportional to bond energy. Shorter bonds result in a higher bond energy. The double bond in ethene is the shortest bond out of all the others. As a result, it has the highest bond energy.
Note that in benzene there are three double bonds and three single bonds between carbons, however, resonance means that each of these only has partial double bond character, and is therefore longer than a pure double bond.
In organic chemistry, the trend is that bond length is inversely proportional to bond energy. Shorter bonds result in a higher bond energy. The double bond in ethene is the shortest bond out of all the others. As a result, it has the highest bond energy.
Note that in benzene there are three double bonds and three single bonds between carbons, however, resonance means that each of these only has partial double bond character, and is therefore longer than a pure double bond.
Compare your answer with the correct one above
The answer is arrows A and C. The carbon that is pointed to by arrow C is  hybridized. We see that its bond angles are at 120º (the full substituent points into the page) with a p-orbital that is involved in the pi bond of the carbonyl. Phenyl rings are also made up of carbons that are all
hybridized. We see that its bond angles are at 120º (the full substituent points into the page) with a p-orbital that is involved in the pi bond of the carbonyl. Phenyl rings are also made up of carbons that are all  hybridized. The nitrogen pointed to by arrow B has two electrons that are not shown, but cause the atom to be
hybridized. The nitrogen pointed to by arrow B has two electrons that are not shown, but cause the atom to be  hybridized. The methyl groups denoted by arrow D are also
hybridized. The methyl groups denoted by arrow D are also  hybridized.
hybridized.
We can quickly tell the hybridization of atoms by observing their double bonds and unbonded electrons. As a rule of thumb, any carbon, nitrogen, or oxygen involved in a double bond will be  hybridized. Any of these atoms with no double bonds will be
hybridized. Any of these atoms with no double bonds will be  hybridized. Finally, nitrogens or carbons involved in triple bonds are
hybridized. Finally, nitrogens or carbons involved in triple bonds are  hybridized.
hybridized.
The answer is arrows A and C. The carbon that is pointed to by arrow C is 



We can quickly tell the hybridization of atoms by observing their double bonds and unbonded electrons. As a rule of thumb, any carbon, nitrogen, or oxygen involved in a double bond will be 


Compare your answer with the correct one above
For a compound to be considered aromatic, it must be flat, cyclic, and conjugated and it must obey Huckel's rule. Huckel's rule states that an aromatic compound must have  pi electrons in the overlapping p orbitals in order to be aromatic (n in this formula represents any integer). Only compounds with 2, 6, 10, 14, . . . pi electrons can be considered aromatic. Compound A has 6 pi electrons, compound B has 4, and compound C has 8. This eliminates answers B and C. Answer D is not cyclic, and therefore cannot be aromatic. The only aromatic compound is answer choice A, which you should recognize as benzene.
pi electrons in the overlapping p orbitals in order to be aromatic (n in this formula represents any integer). Only compounds with 2, 6, 10, 14, . . . pi electrons can be considered aromatic. Compound A has 6 pi electrons, compound B has 4, and compound C has 8. This eliminates answers B and C. Answer D is not cyclic, and therefore cannot be aromatic. The only aromatic compound is answer choice A, which you should recognize as benzene.
For a compound to be considered aromatic, it must be flat, cyclic, and conjugated and it must obey Huckel's rule. Huckel's rule states that an aromatic compound must have 
Compare your answer with the correct one above


 hybridized?
hybridized?


