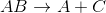All High School Chemistry Resources
Example Questions
Example Question #2 : Types Of Reactions
What is another term for a dissociation reaction?
Single-replacement reaction
Double-replacement reaction
Decomposition reaction
Dehydration reaction
Synthesis reaction
Decomposition reaction
Dissociation reactions occur when one molecule is divided to form two smaller ones, leading to a decrease in energy. Dissociation reactions result in the break down of a large molecule to form smaller products, giving them their second name: decomposition reactions.
Many decomposition reactions can also be classified as hydrolysis reactions, if water is used as a reactant. One example of this is the breakdown of glycogen to form glucose monomers. Dehydration reactions release water in order to form a new bond, building a larger molecule from smaller reactants (synthesis).
Example Question #991 : Ap Chemistry
The reaction above could be classified as __________.
oxidation-reduction
decomposition and oxidation-reduction
single displacement
synthesis
decomposition
decomposition
When one molecule is converted into two or more smaller molecules, the reaction is considered a decomposition reaction. In this example, none of the elements under go a change in oxidation number, so this is not an oxidation-reduction reaction. (Calcium is always 


Example Question #1 : Help With Dissociation Reactions
In a dissociation reaction, determine the potential products for:
Dissociation reactions usually entail a salt or ionic compounds dissociating or splitting into its components. In this case, 
The options that include a third species, would be incorrect, as there are only two species in the original compound that is dissociating.
All High School Chemistry Resources