All Organic Chemistry Resources
Example Questions
Example Question #61 : Isomers

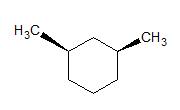
The given molecules are __________.
None of these
stereoisomers
conformers
identical
constitutional isomers
stereoisomers
Stereoisomers have different orientations around a single stereocenter. The two molecules are stereoisomers. Specifically, these molecules are epimers, meaning that they differ at only one stereocenter.
Constitutional isomers have the same molecular formula, but different structures. Conformers have different rotations around a single bond. The molecules are clearly not identical.
Example Question #1 : Help With Epimers
Which of the following carbons represents the stereogenic center between the given isomers?


Carbon 1
Carbon 5
Carbon 3
Carbon 4
Carbon 2
Carbon 4
Epimers are isomers that have different configurations at only one carbon atom. This carbon atom is known as the stereogenic center. The given compounds are identical except for the orientation around carbon number 4; thus, carbon 4 is the stereogenic center.
Example Question #87 : Stereochemistry
Which of these describes an epimer?
Stereoisomers that are not superimposable mirror images
There are no such things as epimers.
One of a pair of stereoisomers, which differ in configuration at only one stereogenic center
Two or more stereoisomers of a compound which have different configurations at one or more (but not all) of the equivalent stereocenters and are not mirror images of each other
One of a pair of stereoisomers, which differ in configuration at only one stereogenic center
In organic chemistry, an epimer refers to one of a pair of stereoisomers, which differ in configuration at only one stereogenic center. Any other stereogenic centers in the compounds are the same in each one. The sugars glucose and galactose are epimers.
Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more, but not all, of the equivalent stereocenters. These stereoisomers are not mirror images of each other. D-erythrose and D-threose are diastereomers.
Note: Epimers are diastereomers that contain more than one stereocenter but differ from each other in the configuration at ONLY one stereocenter. Diastereomers can differ at more than one stereocenter, but not all of them.
Enantiomers are stereoisomers that are non-superimposable mirror images. This means that the molecules cannot be placed on top of one another and give the same molecule. D-threose and L-threose are enantiomers.
Certified Tutor
All Organic Chemistry Resources




