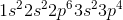All Physical Chemistry Resources
Example Questions
Example Question #1 : Atoms And Elements
How many neutrons are present in the nucleus of one atom of 
From the periodic table, we find that the atomic number, i.e., the number of protons in the nucleus of 


Example Question #1 : Atomic Nucleus
Which atomic symbol represents a period five transition metal that has 42 electrons when it forms a 
Transition metals extend from groups 3 through 12 and periods 4 through 7. Ru, in its neutral state, has 44 electrons. Therefore, when it becomes a 
Example Question #2 : Atoms And Elements
What is the mass number, atomic number, and charge, of the isotope of an atom that contains 34 protons, 36 neutrons, and 36 electrons?
Mass number:
Atomic number:
Charge:
Mass number:
Atomic number:
Charge:
Mass number:
Atomic number:
Charge:
Mass number:
Atomic number:
Charge:
Mass number:
Atomic number:
Charge:
Mass number:
Atomic number:
Charge:
The atomic number is equal to the number of protons in the element, so from the periodic table, we find that the element with atomic number 34 is selenium. Since 

Example Question #1 : Atoms And Elements
Which of the following organizes the forces from strongest to weakest?
Van der Waals forces, dipole-dipole interactions, hydrogen bonding, covalent bonding
Dipole-dipole interactions, hydrogen bonding, covalent bonding, van der Waals forces
Van der Waals forces, hydrogen bonding, covalent bonding, dipole-dipole interactions
Hydrogen bonding, covalent bonding, dipole-dipole interactions, van der Waals forces
Covalent bonding, hydrogen bonding, dipole-dipole interactions, van der Waals forces
Covalent bonding, hydrogen bonding, dipole-dipole interactions, van der Waals forces
Covalent bonds are by far the strongest, requiring 
Next are hydrogen bonds, which require between 
Next are dipole-dipole interactions, which require 
Finally, van der Waals forces are the weakest of those listed, requiring 
Example Question #1 : Electron Configuration
Which of the following electron configurations indicates an atom in an excited state?
An atom is considered to be in an excited state when one of the electrons has jumped to a higher energy level while a lower energy level is available. In the case of 
Example Question #91 : Physical Chemistry
What is the complete ground state electron configuration for the magnesium atom?
1s42p63s2
1s22s22p23s2
1s22s22p63s6
1s22s23s2
1s22s22p63s2
1s22s22p63s2
Magnesium has an atomic number of 12, so the total number of electrons in its configuration should add up to twelve. The maximum number of electrons in the s subshell is two. Of all the answer choices, only 1s22s22p63s2 fits the criteria. The sum of the exponent values is 12, matching the atomic number of magnesium, and the number of electrons in the s and p subshells matches the maximum amount possible.
Example Question #1 : Electron Configuration
What is the complete ground state electron configuration for the iron atom?
1s22s22p63s23p63d6
1s22s22p63s23p64s23d6
1s42s22p63s23p24s23d6
1s22s22p63s23p64s23d4
1s22s22p63s24s23d6
1s22s22p63s23p64s23d6
Iron has an atomic number of 26, so the total number of electrons in its configuration should add up to twenty six. The maximum number of electrons in the s subshell is two. The sum of the exponent values is 26, matching the atomic number of magnesium, and the number of electrons in the s and p subshells matches the maximum amount possible.
Example Question #1 : Atoms And Elements
In an atom or molecule, why can't two electrons have the same four electronic quantum numbers?
Harmonic Reaction Orders
The Pauli Exclusion Principle
Kinetic energy operator
Heisenberg Uncertainty Principle
The first law of thermodynamics
The Pauli Exclusion Principle
The Pauli Exclusion Principle explains various phenomena such as the structure of atoms and how different atoms combine to share electrons. When you have two electrons that are located in the same orbital, the quantum numbers n, l and ml are the same. However, ms will be different. Two electrons cannot have the same four electronic quantum numbers because no more than two electrons may occupy an orbital, and if they do, the spin of one must cancel the spin of the other so their spins will have a zero net spin angular momentum.
Example Question #1 : Atoms And Elements
What is the hybridization on the nitrogen atom in a molecule of ammonia?
sp3d
sp2
sp3
sp
sp3
The hybridization of an atom can be determined by the number of atoms it is bonded to, as well as the number of lone pairs it has. Two of these variables would be sp, three variables would be sp2, and four would be sp3.
The nitrogen in ammonia is bonded to three atoms of hydrogen, but also has a lone pair in order to satisfy its octet. This means that nitrogen exhibits sp3 hybridization.
Example Question #4 : Atoms And Elements
Which of the following are true regarding 

I. Both 

II. In a given shell, 
III. 

III only
I only
I and III
I and II
I and III
Orbitals are regions in an electron shell where electrons might be located. There are several types of orbitals such as 










Certified Tutor
Certified Tutor
All Physical Chemistry Resources