All AP Chemistry Resources
Example Questions
Example Question #1 : Balancing Chemical Equations
What is the formula for the dissociation of iron (II) phosphate?
Iron (II) has a positive two charge: 
Phosphate has a negative three charge: 
The initial compound must be constructed to cancel these charges. The dissociation is: 
Example Question #1 : Solubility
Pb(OH)2 has a Ksp of 1.43 * 10-20.
What is its solubility?
1.53 * 10-7M
2.43 * 10-7M
1.20 * 10-10M
8.42 * 10-12M
1.53 * 10-7M
Lead (II) hydroxide dissociates according to this reaction: 
Ksp = [Pb2+][OH-]2
If [Pb2+] = x, then [OH-] = 2x. They exist in a 1:2 ratio.
Ksp = 1.43 * 10-20 = x(2x)2 = 4x3
x = 1.53 x 10-7M
Example Question #2 : Solubility
Which of the following factors will generally lead a solute to dissolve more easily into a solution (i.e. increase solubility)?
Increasing the temperature of the solution
None of these choices will increase solubility
Decreasing the temperature of the solution
The common ion effect
Increasing the temperature of the solution
Increasing the temperature of a solution will generally increase its solubility, while decreasing temperature will have the opposite effect. The common ion effect refers to the phenomenon when two compunds in a solution dissociate to produce at least one similar ion, and will make it harder for additional amounts of that ion in the solution to dissociate, decreasing solubility.
Example Question #3 : Solubility
If the Ksp of the above dissociation is found to be 2.70 * 10-4, what is the solubility of NaCl?
7.29 * 10-8M
1.44 * 10-2M
1.89 * 10-2M
1.64 * 10-2M
1.64 * 10-2M
First, set up a balanced reaction and enter X for the amount of each solute that is going to be dissolved. As NaCl dissociates, each ion amount will increase by some amount, X.




Then set up the equation for the value of Ksp and enter in the value of X for each solute. Use this equation to solve for X, the solubility (in mol/L). Remember that NaCl si not included in the equation, as it is a pure solid.
Example Question #1 : Solubility
If the solubility of CaF2 in the dissociation reaction above was determined to be 1.54 * 10-3M, what is the Ksp value for CaF2?
1.98 * 10-8
1.46 * 10-8
1.42 * 10-6
2.24 * 10-6
1.46 * 10-8
Use the balanced reaction of the dissociation to assign values of X for the solubility of each ion. As CaF2 dissociates, each ion amount will increase by some amount; calcium will increase by X and flouride will increase by 2X.




Then plug in the equation to solve Ksp, using the X values assigned for each solute. Use the given value for solubility to find Ksp. Remember that NaCl si not included in the equation, as it is a pure solid.
Example Question #1 : Solubility
Consider the following balanced equation for the solubility of barium hydroxide in an aqueous solution.
What is the solubility of barium hydroxide?
In order to solve for the solubility of barium hydroxide, we need to establish an ICE table for the reaction.
I. Initially, there are no barium ions or hydroxide ions in the solution, so we can call their concentrations zero at the beginning of the reaction.
C. For every molecule of dissolved barium hydroxide, there will be one ion of barium and two ions of hydroxide. As a result, the increases in each ion can be designated 

E. Finally, we plug these values into the equilibrium expression and set it equal to the solubility product constant.
Example Question #6 : Solubility
Four different insoluble salts are added to an aqueous solution in equal amounts. Assume that the salts do not interact with one another in any way. The following list shows the salts and their solubility product constants.



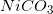
Which of the following ions will have the highest concentration in the solution?
The solubility product constants help us envision which ions will have the largest concentrations after dissolving to equilibrium. 
Upon the dissolving of 

Example Question #3 : Solubility
Consider the following balanced equation for the dissociation of barium hydroxide in an aqueous solution.
A 1M solution of barium nitrate is added to the solution. What is the solubility of barium hydroxide after this addition?
Before we solve for the solubility, remember Le Chatelier's principle. Barium nitrate is a soluble salt that will completely dissolve in solution. This means that there will be a one molar concentration of barium ions before the salt dissolves. Since we have added to the products side of the reaction, we can predict that the equilibrium will be shifted to the left, and the barium hydroxide will be less soluble. This change in equilibrium is referred to as the common ion effect.
We will use an ICE table to determine the solubility of the salt.
I. Before the salt begins to dissolve, there is a one molar concentration of barium ions (from the barium nitrate) with no hydroxide ions in the solution.
C. Since every dissolved molecule of barium hydroxide will yield one barium ion and two hydroxide ions, the unknown increases in each ion can be designated 

E. Now, we set the solubility expression equal to the solubility product constant.
Since 1 is such a larger number than the value we will get for 
The solubility of barium hydroxide without the common ion effect is 0.108M. Notice how the solubility for the salt has decreased, as was predicted in the beginning by Le Chatelier's principle.
Example Question #4 : Solubility
A chemist has a fully saturated solution of chemical X, with solid X resting on the bottom. If she adds a chemical that will react with the aqueous X, what will happen to the solid?
The solid will change color
More solid will precipitate out of solution
It will start to dissolve into the solution
A layer of sediment will cover the solid
Nothing will change
It will start to dissolve into the solution
A fully saturated solution is at equilibrium between the solid and aqueous states, but it is a dynamic equilibrium. As the reacting chemical reacts with aqueous X, more and more of the solid will dissolve into solution.
Example Question #5 : Solubility
The partial pressure of an arbitrary gas, 
The Henry's Law constant for 

What is the solubility of 
None of the available answers
Henry's law can be expressed:
Where 

Certified Tutor
All AP Chemistry Resources










![K_{sp}= [Na^{+}][Cl^{-}] = (X)(X) = X^{2}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/85510/gif.latex)






![K_{sp}= [Ca^{2+}][F^-]^2=(X)(2X)^{2} = 4X^{3}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/119041/gif.latex)








![K_{sp}=[Ba^{2+}][OH^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94058/gif.latex)
![\small 5*10^{-3} = [x][2x]^{2}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/126927/gif.latex)






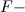



![[F^-]<[CrO_4^{2-}]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/119420/gif.latex)








![K_{sp}=[Ba^{2+}][OH^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/110662/gif.latex)
![\small 5*10^{-3} = [1+x][2x]^{2}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94077/gif.latex)
![\small 5*10^{-3} = [1][2x]^{2}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94078/gif.latex)










