The Varsity Tutors High School Chemistry Mobile App
Many high school students are required to take a class in chemistry, which covers a wide range of concepts that can easily trip the best student up. They’ll learn about ionic compounds, acids, nonpolar bonds, bases, and so much more over the course of the school year. With the free Varsity Tutors High School Chemistry app, students are able to access a wide range of foundational chemistry materials alongside thorough explanations, practice tests, and more. The app offers innovative tools that allow students and parents to work together to form a study plan that truly helps the student by avoiding repeating the information they are already familiar with.
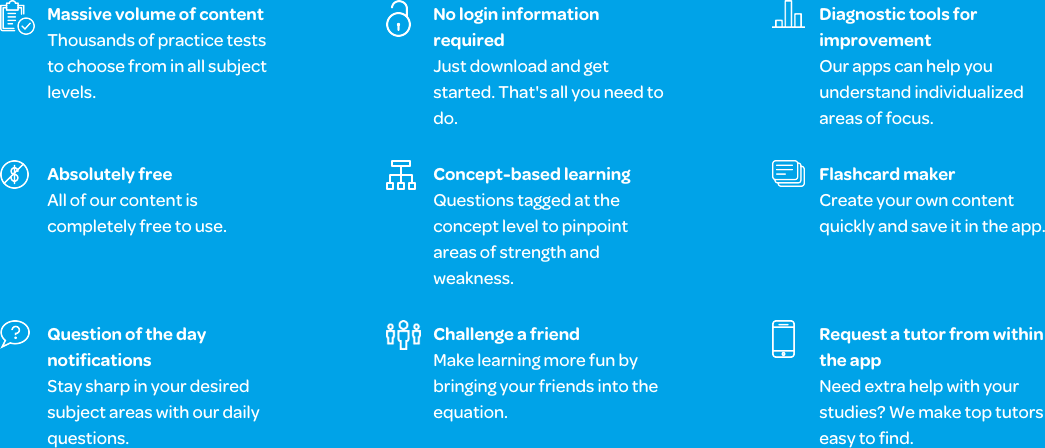
The app offers easy-to-follow explanations that cover a wide range of concepts. Students can access the chemical reactions section, for instance, to learn about balancing reactions, equilibrium, double-replacement, oxidation-reduction reactions, or dissociation, among many others. They can then take a concept-specific practice test to evaluate their abilities in a particular area. Students can also take full-length practice tests. The full-length exams cover everything that is taught during high school chemistry to assist them as they prepare for the final. In addition, the app can serve as a supplemental resource to accompany students’ ongoing coursework or a study tool for upcoming unit exams.
The app also provides students with access to the Learn by Concept tool. This tool provides hundreds of unique, concept-specific practice materials. The questions’ answers are altered and rearranged each time the student interacts with them, ensuring that the experience remains challenging. Each question provides a detailed explanation for the correct answer, which allows students to gain a deeper understanding of the concepts behind the answer. Taking full advantage of these features can enhance their study sessions.
Students can also track and share their progress in the app’s Tests Taken feature, which allows them to easily send their test scores to family members, teachers, and tutors. The feature also offers a review of the student’s performance by concept, breaking down which questions the student answered successfully and which could be improved upon. These features allow them to assess their readiness on their own, with the guidance of a teacher or parent, or with a study partner.
There are hundreds of high school chemistry flashcards on the app, and students can also create their own using the app’s adaptive Flashcard Maker tool. These are great for quick study sessions or for use with a study buddy. Each student can remove the concepts they are already familiar with to focus on more challenging concepts. Some students may even prefer the fast-paced flexibility offered by this feature. In addition, there is an ever-changing Question of the Day available for each section of high school chemistry. The question is pulled from the practice exams at random. This feature also provides a helpful review of the student’s time and results, showing the comparison between their results and the average of other students who have answered the same question.
For an easy, convenient mobile studying experience, try the Varsity Tutors High School Chemistry app. It’s available from iTunes and the Google Play Store. Enhance your study sessions by downloading the app today!
66 mobile apps to choose from for your tutoring needs.
Learn More
As a high school student with aspirations for a science or engineering degree in college, or if you just take an interest in science for fun, you may find yourself in a high school chemistry course. While the curriculum may vary depending on school districts, each high school chemistry course should include the same basic fundamentals of chemistry.
A high school chemistry course is probably your first time formally studying chemistry. However, you probably already have a basic knowledge of chemical reactions, and you may not even know it. As a chemistry student, you will develop a deeper understanding of matter and how atoms interact, and of elements and compounds, which will be achieved through lectures and hands-on experiments.
As you begin to develop an understanding of chemistry, you will learn about chemical reactions, as well as the properties, structure, and composition of matter. Your introduction to chemistry will include the study of atomic number and mass, atoms, isotopes, and elements. You will learn how all matter is made up of atoms, as well as the subatomic particles that make each atom. Additionally, you will learn about 118 of the elements on the periodic table, ionic and covalent compounds, and how molecular mass and weight are calculated.
One of the first things you will learn in chemistry is the difference between an acid and a base, and what it means for something to have corrosive properties. You will learn about the pH scale and chemical equilibrium. In this section, you will also learn about the Bronsted-Lowry acid base theory and how to explain what makes something acidic or basic.
As you develop an understanding of acids and bases, you will start learning about the important role that buffer solutions play in regulating pH balance. Additionally, you will learn about how titrations are used to evaluate the concentration of an acid or base, and how you can calculate the pH level of a variety of substances.
Another important component of high school chemistry is understanding chemical reactions. This area is arguably one of the most important aspects of chemistry, because in this section you will learn about the various ways that molecules react. Before you dig deeper into chemical reactions, however, you must first learn how to ensure that your reaction and reactants both have the same amount of atoms. This is called “balancing chemical equations.”
After understanding the importance of equilibrium and balancing chemical equations, you will begin to learn about various types of chemical reactions. This includes learning about how chemical reactions are the result of atoms being combined, rearranged, or separated. You will also learn about the following types of reactions: dissociation reactions, double-replacement, oxidation-reduction, single-replacement, and addition reactions.
As you learn about oxidation-reduction reactions, you will cover how these reactions play a role in many aspects of the real world, from helping your body absorb oxygen to powering electronic devices. This subtopic is called electrochemistry, and it focuses on the chemical processes that force electrons to move, thus creating electricity. You will also learn about electrolytic cells, and how to compare these cells to voltaic cells. Moreover, you will learn about galvanic cells, and how to apply the Nernst equation as a way to determine the cell potential for a concentration cell.
Part of understanding chemistry is evaluating how fast a reaction can occur, and what external factors can be presented to influence the reaction rate. This study is referred to as kinetics, and it is an important component in high school chemistry. For you to be able to determine the order of a reaction, you must understand the following concepts: kinetics, activation energy, catalysts, and activated complex. You should also be familiar with understanding the ways that activation energy and temperature influence reaction rates.
Another important topic you will learn about is nuclear chemistry, and how nuclear reactions can alter the makeup of an atom’s nucleus. A central concept relating to this field is nuclear decay, of which there are four types: alpha, beta, gamma, and positron emission. Furthermore, you will become familiar with carbon dating, and how it is an example of a first-order reaction.
As you further your understanding of atoms and molecules, you will also learn the various states of matter. You should be aware that matter exists in the four forms: solid, gas, plasma, and liquid. It is important that you learn the properties of each one of these states of matter, and be able to explain how something can change from one state to another. These transitions include melting, freezing, condensation, and vaporization.
When you move into the topic of solutions and mixtures, you’ll learn to understand what the difference between a solvent, solute, and solution is. You will learn that solubility is the amount of solute required to make a saturated solution, and you’ll need to taught the necessary steps required to calculate solubility in exams.
The next major topic you’ll cover is stoichiometry, which is the part of chemistry that handles relationships between reactants and the products of a chemical reaction. As part of this, you will have the opportunity to understand molar mass and mole ratios.
You will also learn about thermochemistry and the laws of thermodynamics. These laws relate to kinetic energy, potential energy, and electricity, and they are important concepts. You should also be familiar with enthalpy change, or how energy has changed during the course of a chemical reaction. Additionally, entropy, which is the process of measuring randomness in a thermodynamic system, is also an important concept to understand. Finally, you’ll have the opportunity to develop a working knowledge of endothermic and exothermic reactions, learning about which process causes systems to gain or lose heat.
While chemistry may appear to be overwhelming, you should be able to complete the course successfully if you implement the right study habits. By understanding these concepts, definitions, and theories, you are working to develop a greater understanding of the fundamentals of chemistry, which can prove useful on exams now, and for college courses in the future.




