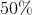All AP Chemistry Resources
Example Questions
Example Question #1 : Yield And Error
3 mols of 


What is the percent conversion of 
To find the percent conversion, use the following equation:
Initially there are 3 mols of 



Plugging this into the above equation gives a conversion rate of 58%.
Example Question #211 : Ap Chemistry
You run an experiment in order to empirically find the gas constant, 
You know from the literature that
What is the percent error in your experimental value of 
To find the percent error in your 
Doing this, we find the percent error to be 4.6%.
Example Question #35 : Calculations
Suppose that a chemist working in a lab is trying to synthesize caffeine. In doing so, he predicts that he can produce 

When chemists synthesize compounds in the lab, a theoretical yield can be calculated by using knowledge of the full, balanced chemical equation, as well as the starting amounts of all reagents including the limiting reagent. However, due to a variety of factors, the actual yield obtained at the end of the experiment is nearly always going to be lower than the theoretical yield. Some of the things that can cause this are experimental error and side reactions, among others. Thus, chemists usually take the ratio of actual yield to theoretical yield to give the percent yield.
This means that the overall process was 
Certified Tutor
All AP Chemistry Resources