All MCAT Biology Resources
Example Questions
Example Question #1 : Functional Groups And Properties
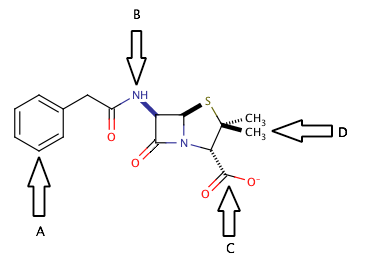
Shown above is the chemical structure for penicillin. Which of the arrows is pointing to an amide group?
D
A
B
C
B
The correct answer is arrow B. An amide group is a carbonyl group with a nitrogen that is in the alpha position (directly attached to the carbonyl carbon). Penicillin has two different amide groups in its chemical structure, but only one has an arrow pointing to it in the diagram. Arrow B points to a primary secondary amide, bond to one hydrogen and two carbons. The other amide in the molecule is a tertiary amide, bond to three carbons and no hydrogens.
Example Question #1731 : Mcat Biological Sciences
Which of the following statements about amide bonds is NOT true?
Amides are formed by the combination of a carboxylic acid and an amine, resulting in the loss of water
The nitrogen is 

Amide bonds are polar
Amides are mildly acidic, with primary amides having a pKa of approximately fifteen
A secondary amide has the following structure.

The nitrogen is 

Amides have a structure of a ketone group adjacent to an amine group.
In an amide bond, all of the atoms (the nitrogen, the carbonyl oxygen, and carbonyl carbon) are 
Example Question #1 : Functional Groups And Properties
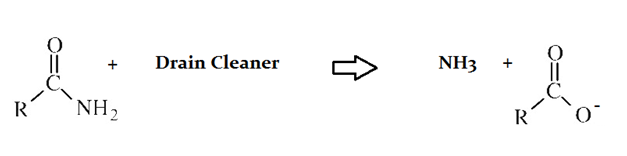
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The NH4 molecule produced in Reaction 2 must have __________.
a negative charge, since one was also created on the other product
an expanded octet for the N atom
a positive charge due to the presence of an extra hydrogen
no charge, since N has a lone pair of electrons available to bond
a basic nature due to the presence of the nitrogen atom
a positive charge due to the presence of an extra hydrogen
The additional H present on ammonia (NH3) requires the generation of the a positive charge on the molecule. This also must be created to balance the negative charge created on the associated anion product.
Example Question #2 : Functional Groups And Properties
Which of the following amines is the strongest base?
A primary amine with an electron-donating functional group
A secondary amine with electron-withdrawing functional groups
Ammonia
A secondary amine with electron-donating functional groups
A secondary amine with electron-donating functional groups
Amines act as bases due to the lone pair on the nitrogen. Amine basicity can be increased or decreased by functional groups attached to the nitrogen. Electron-withdrawing groups decrease the basicity of an amine by lessening the effect of the lone pair, while electron-donating groups increase basicity by amplifying the lone pair effect. As a result, a secondary amine with an electron-donating functional groups will be the most basic amine.
Example Question #31 : Organic Functional Groups
Ephedrine (shown below) contains what type of amine?

Primary
Secondary
Quaternary
Tertiary
Neutral
Secondary
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Example Question #1 : Functional Groups And Properties
Your lab isolates a compound with the formula 
Nitrobenzene
Benzylamine
Benzylamide
Benzylmethylamine
None of these
Benzylamine
Nitro groups and amide groups both contain oxygen components, and cannot be found in the compound described. We also know that the benzene ring only has a single constituent, meaning that it cannot be a methylamine. The compound must be benzylamine, a benzene ring with a -CH2NH2 substituent.
Example Question #3 : Functional Groups And Properties
What is the name of the functional group that contains a vinylic hydroxyl group?
Enol
Carboxyl
Keto
Aldehyde
Enol
Enol functional groups contain a hydroxyl group bonded to a carbon involved in a double bond with another carbon (vinyl carbon). An enol will usually undergo tautomerization to become a more stable keto.
Example Question #1 : Functional Groups And Properties
Ephedrine, whose structure is shown below, is used commonly as a stimulant and decongestant.

Ephedrine contains all of the following functional groups except __________.
arene
amine
ketone
alcohol
N-methyl group
ketone
Ephedrine contains an arene (the aromatic benzene ring), an alcohol (the -OH group), an amine (the nitrogen-based group), and an N-methyl group (-CH3 attached to nitrogen). It does not contain a ketone (C=O), or any other carboxyl groups.
Example Question #1 : Nucleophilic Addition
Which of the following compounds would you expect to undergo a nucleophilic addition reaction?
Acetic acid
Methyl ethanoate
Propanal
Ethanamide
Propanal
When dealing with carbonyl compounds, remember that a carboxylic acid and all of its derivatives will undergo nucleophilic substitution. Aldehydes and ketones will undergo nucleophilic addition. Propanal is a three-carbon aldehyde, and will thus undergo nucleophilic addition.
Acetic acid is a carboxylic acid, methyl ethanoate is an ether, and ethanamide is an amide; each of these would undergo nucleophilic substitution.
Example Question #4 : Functional Groups And Properties
Compound A, shown below, contains an example of what type of functional group?

Ketone
Nitrile
Carboxylic acid
Ether
Ester
Ester
Esters have the general molecular formula of 


Ketones have the formula of 



Compound A also contains an aromatic function group (the benzene ring) and a nitro group, 
Certified Tutor
All MCAT Biology Resources