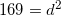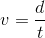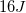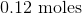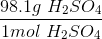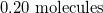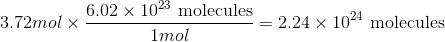All GED Science Resources
Example Questions
Example Question #1 : Motion And Mechanics
Which of the following best illustrates Newton's Second Law?
If an object is not accelerating, then the net force on it is zero
A ball is rolled across a table and does not stop until it hits an area with friction
When two cymbals collide, some kinetic energy is converted into sound
As a hammer hits a nail, the nail also exerts a force on the hammer
If an object is not accelerating, then the net force on it is zero
Newton's Second Law is best represented as an equation, in which the product of mass and acceleration is equal to net force:
By this principle, if there is zero acceleration, then the force must also be zero.
A non-zero acceleration must be present if there is a non-zero net force.
Example Question #2 : Motion And Mechanics
Which of the following is the best example of Newton's Third Law?
When two objects collide, the total initial and total final momentum will be equal
A spinning top will not fall unless there is friction with the surface on which it spins
If an object's weight rests on the floor, then the floor will exert a normal force on the object
The force of being hit by an object is determined by its mass and acceleration
If an object's weight rests on the floor, then the floor will exert a normal force on the object
Newton's Third Law states that for every action there is an equal and opposite reaction. In other words, a force will act in equal and opposite directions on the two objects involved.
Weight and normal force are a specific example of this principle. Acceleration due to gravity creates a downward force, known as weight. This force, however, results in zero downward acceleration when the object is resting on a surface (a book does not fall through a table, for example). For the forces to be in equilibrium, there must be a counteracting upward force. This is known as the normal force, which is equal and opposite to the weight of the object. While weight pulls the object down, normal force pushes it up to create equilibrium.
Example Question #1 : Mechanics Principles
A car drives 

The car has covered a total distance of 
To solve this question, it is best to draw a sketch. The resulting sketch will be a right triangle with the hypotenuse equal to the displacement. The Pythagorean Theorem can be used to solve the problem.

Example Question #2 : Mechanics Principles
A car travels due east for two hours and covers 144km. What is the car's average velocity in meters per second?
This question requires us to convert between units, then solve for the velocity.
First, convert kilometers to meters. There are 1000m in 1km.
Then, convert hours to seconds. There are 60min in 1hr, and 60sec in 1min. In other words, there are 3600s in 1hr.
Now that you have both the distance in meters and the time in seconds, you can find the velocity. Velocity is equal to the distance divided by the time.
Example Question #1 : Physics
A ball that has a mass of 2kg is launched upward at a velocity of 
Assume the ball is traveling in a vacuum.
Kinetic energy (measured in Joules) can be solved using the equation:
We are given both the mass of the projectile (2kg) and its velocity (
Example Question #2 : Physics
Which of the following measures depicts the amplitude?

A
C
D
B
A
The amplitude measures the greatest displacement (either positive or negative) of a wave from the x-axis. Visually, the amplitude appears to be the distance from the x-axis (center line) to the top of one peak. In the figure, this value is given by A.
The other relevant measure given in the figure is segment D, which measures the distance between two peaks. This distance is known as the wavelength.
Segments B and C do not provide useful measurements of the wave function.
Example Question #1 : Ged Science
Molecules are constantly moving. In which state of matter do molecules move the fastest?
Liquids
Gases
Molecules move at the same rate in both gases and liquids
Solids
Molecules move at the same rate in all three states of matter
Gases
The movement of particles is important in differentiating the states of matter. Solids have the lowest molecular energy; their molecules barely vibrate and are generally trapped in lattice structures. Molecules in liquids are able to move more freely, resulting in the fluidity of the liquid state. Gas molecules have even more freedom of movement, allowing the gas to expand to fill a space.
Consider that energy (such as heat) must be added to convert a solid to a liquid or a liquid to a gas. That energy goes to make the molecules move faster and increase the temperature of the compound. Gases require the most energy (consider boiling water), and will have the fastest moving molecules.
Example Question #2 : Chemistry
Atomic mass values:
Convert the following mass to moles:
To convert grams to moles, use the molecular weight of the molecule. This is found by adding the mass of each atom.
Now we can use the molecular mass to convert the given mass in the question to moles. Make sure to align the values so that the units cancel out.
Example Question #2 : Ged Science
How many molecules are in 3.72mol of fluorine?
Convert from moles to molecules using Avogadro's number:
Use the moles given in the question to solve.
Example Question #3 : Ged Science
Which of the following types of chemical bonds is very strong and involves transfer of an electron between atoms?
Disulfide bonds
Hydrogen bonds
Ionic bonds
Covalent bonds
Ionic bonds
There are two primary bond types: ionic bonds and covalent bonds. Ionic bonds involve the transfer of an electron between atoms, while covalent bonds require atoms to share an electron between them. Ionic bonds are the stronger of the two. Ionic bonds usually form between a metal and a non-metal (for example, NaCl), while covalent bonds usually form between two non-metals (for example, HCl).
Hydrogen bonds are a type of intermolecular force and are similar to a very weak covalent bond. Hydrogen bonds are not actual bonds, and do not chemically link atoms together; they are strictly physical interactions. Disulfide bonds are covalent bonds between sulfur atoms that help form protein structure.
Certified Tutor
All GED Science Resources