All MCAT Biology Resources
Example Questions
Example Question #1 : Enzymes And Enzyme Inhibition
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
Carbonic anhydrase is an organic enzyme. Which of the following is true of carbonic anhydrase? Assume no CO2 or bicarbonate is lost in the reaction.
I. It lowers the activation energy for the conversion of carbon dioxide to carbonic acid
II. It shifts the equilibrium toward carbon dioxide in experimental conditions
III. It modifies chemical species at its allosteric site
I and III
I and II
I, II, and III
III, only
I, only
I, only
Only choice I is correct. Carbonic anhydrase lowers the activation energy of a chemical reaction, as does any catalyst. Thermodynamics, including equilibria, are not modified by catalysts, so choice II is incorrect. Choice III is also incorrect, as an allosteric site is typically used to bind regulators of enzymes to induce conformational changes, while an active site would be where the actual catalysis takes place.
Example Question #2 : Enzymes And Enzyme Inhibition
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be optimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
The observed cooperativity of oxygen binding to hemoglobin can be explained by changes in shape to the hemoglobin molecule upon oxygen attachment. What kind of change would this be considered?
Primary
Allosteric
Uncompetitive
Noncompetitive
Competitive
Allosteric
Hemoglobin reacts with an allosteric change to oxygen binding, because the shape of the molecule changes. In fact, oxygen is considered a "homotropic" allosteric regulator because it is the normal substrate for hemoglobin, and affects its changes on that molecule by binding to its active site.
Example Question #3 : Enzymes And Enzyme Inhibition
Cryptosporidium is a genus of gastrointestinal parasite that infects the intestinal epithelium of mammals. Cryptosporidium is water-borne, and is an apicomplexan parasite. This phylum also includes Plasmodium, Babesia, and Toxoplasma.
Apicomplexans are unique due to their apicoplast, an apical organelle that helps penetrate mammalian epithelium. In the case of cryptosporidium, there is an interaction between the surface proteins of mammalian epithelial tissue and those of the apical portion of the cryptosporidium infective stage, or oocyst. A scientist is conducting an experiment to test the hypothesis that the oocyst secretes a peptide compound that neutralizes intestinal defense cells. These defense cells are resident in the intestinal epithelium, and defend the tissue by phagocytizing the oocysts.
She sets up the following experiment:
As the neutralizing compound was believed to be secreted by the oocyst, the scientist collected oocysts onto growth media. The oocysts were grown among intestinal epithelial cells, and then the media was collected. The media was then added to another plate where Toxoplasma gondii was growing with intestinal epithelial cells. A second plate of Toxoplasma gondii was grown with the same type of intestinal epithelium, but no oocyst-sourced media was added.
Where is the likely site of the neutralizing toxin synthesis in cryptosporidium cells?
Nucleus
Ribosomes
Mitochondria
Nucleolus
Smooth endoplasmic reticulum
Ribosomes
The passage specifies that the neutralizing agent is a peptide. Ribosomes synthesize peptides. Nuceloulus may have been a tempting answer, but is where ribosomes are synthesized, not peptides.
Example Question #212 : Organic Chemistry, Biochemistry, And Metabolism
Of the following statements, which is true regarding the change in free energy (ΔG) of a reaction?
When ΔG is zero, the system is at equilibrium.
ΔG is a measure of whether a reaction is spontaneous.
ΔG predicts the rate of a reaction.
Two of these answers are correct.
Two of these answers are correct.
Gibbs Free Energy (G) is a measure of the capacity of a system to do useful work as it proceeds to equilibrium. ΔG measures the spontaneity of a reaction; a negative value for ΔG indicates a spontaneous reaction, a positive value indicates a non-spontaneous reaction, and a value of zero indicates a reaction at equilibrium. ΔG does not predict enzyme kinetics; it only predicts thermodynamics, thus, two of the answers are correct.
Example Question #4 : Enzymes And Enzyme Inhibition
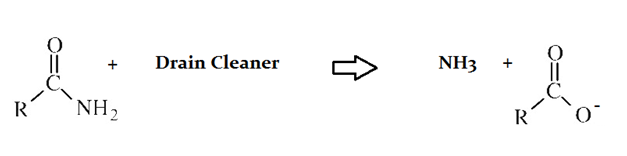
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Protein that forms the hair discussed in the preceeding passage is considered strucutral protein. Functional proteins, such as enzymes, are the other major class. Which of the following is true of enzymes?
They lower activation energy by providing an alternate reaction mechanism.
They only lower activation energy of the original reaction mechanism.
They raise activaiton energy to prevent the reverse reaction from occuring as quickly.
They lower activation energy by changing the equilibrium position of a reaction.
They lower activation energy by changing the products of a reaction.
They lower activation energy by providing an alternate reaction mechanism.
Enzymes are biological catalysts that function to lower activation energy via an alternative reaction pathway. They never alter the equilibrium of the reaction they impact.
Example Question #5 : Enzymes And Enzyme Inhibition
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Protein that forms the hair discussed in the preceeding passage is considered strucutral protein. Functional proteins, such as enzymes, are the other major class. Which of the following is expected in an enzymatic biological reaction?
I. Faster rate than non-enzymatic reaction
II. Enzymatic coupling to hydrolysis reactions
III. More product generation relative to amount of reactant than non-enzymatic reaction
I and II
II, only
I, only
I and III, only
I, II, and III
I and II
Enzymatic reactions will always proceed faster than if there was no enzyme present. They will also often be coupled to hydrolysis reactions to drive them forward thermodynamically, such as ATP hyrodlysis to make an otherwise unfavorable reaction proceed. The equilibrium constant of an enzymatic reaction is never different than the constant for the same reaction without enyzme, however, and thus choice III is incorrect.
Example Question #1 : Enzymes And Enzyme Inhibition
A student observes an enzymatic chemical reaction that normally takes place in human blood. She performs an experiment to see how certain conditions affect the reaction with the enzyme fully saturated with substrate. What should she do to speed the reaction up?
Remove some of the enzyme
Increase the temperature to 40 degrees Celsius
Add more substrate
Increase the enzyme concentration
Make the pH 7.0
Increase the enzyme concentration
Adding more enzyme is the only way to make this reaction proceed faster. Since this is a reaction that takes place in the blood, the optimal conditions are 37 degrees Celsius and a pH of 7.4. Adding more substrate could help in certain conditions, but we know from the question that there is no free enzyme in the reaction so adding more would not help. Removing enzyme would obviously sow the reaction down.
Example Question #2 : Enzymes And Enzyme Inhibition
In order to catalyze a reaction, an enzyme is required to __________.
decrease the activation energy
increase the activation energy
be saturated with substrate
increase the equilibrium constant
decrease the activation energy
Enzymes are biological catalysts that are responsible for the acceleration of the rate and specificity of many metabolic reactions. In order for rate acceleration to occur, an enzyme lowers the activation energy of a reaction. This allows for products to be formed more quickly and reactions to reach equilibrium more rapidly. Substrates bind to the active site of an enzyme and, in the presence of a large concentration of substrate, enzyme active sites become saturated and the reaction rate reaches a maximum constant. The equilibrium constant is calculated from the expression for chemical equilibrium, and is not affected by enzymes. Thus, the correct answer is to decrease the activation energy.
Example Question #51 : Macromolecules
Fetal hemoglobin has a higher binding affinity for oxygen than does adult hemoglobin.
In comparison to the adult oxyhemoglobin dissociation curve, the fetal oxyhemoglobin dissociation curve will __________.
be shifted to the right and display a lower Km
be shifted to the right and display a higher Km
be shifted to the left and display a higher Km
be shifted to the left and display a lower Km
be shifted to the left and display a lower Km
Fetal hemoglobin is associated with a left-shift due to its greater binding affinity for oxygen. The Michaelis constant, Km, is defined as the substrate concentration at which the reaction rate is 0.5 * Vmax. A low Km indicates high substrate affinity.
Example Question #1611 : Mcat Biological Sciences
Which of the following is NOT a class of enzyme?
Pyrimidine complex
Transferase
Hydrolase
Ligase
Isomerase
Pyrimidine complex
The correct answer is pyrimidine complex. A pyrimidine refers to a type of nucleotide base. Enzymes commonly have the suffix -ase at the end of their name.
Certified Tutor
All MCAT Biology Resources