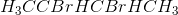All Organic Chemistry Resources
Example Questions
Example Question #511 : Organic Chemistry
2-butene reacts with 
No reaction
Here we have a classic electrophilic addition reaction.
We must remember that a double bond is an area in a molecule that has a high electron density. As a result, double bonds can act as nucleophiles and react with good electrophiles.
In this problem, the alkene attacks the dibromide to form a bromonium ion, which is rather unstable. The bromonium ion pulls carbon's electrons toward itself, causing the carbons in the bromonium complex to have a slightly negative charge. As a result, the negatively charged bromine can attack either of the carbon atoms and, thus, we have added two bromines to our carbon chain.
Example Question #1 : Other Reaction Mechanisms
How many stable resonance structures are there for the following molecule (include the given structure in your total count)?

5
6
3
1
4
5
The five resonance structures of the given molecule are shown below:

Example Question #2 : Other Reaction Mechanisms
Rank the following compounds in order of increasing rate of electrophilic aromatic substitution.

V, I, III, IV, II
II, I, III, IV, V
V, II, I, III, IV
II, IV, III, I, V
IV, V, I, III, II
V, I, III, IV, II
The nitro substituent is strongly deactivating, ketones are moderately deactivating substituent, halides are weakly deactivating, alkyl groups are weakly activating, and amine groups are strongly activating.
Certified Tutor
All Organic Chemistry Resources