All Organic Chemistry Resources
Example Questions
Example Question #1 : How To Quantify Compounds
A chemist places a sample into a mass spectrometer and obtains the following spectrum that features a parent ion of 122 m/z as shown below. What element, in addition to carbon and hydrogen, must be present in her sample?
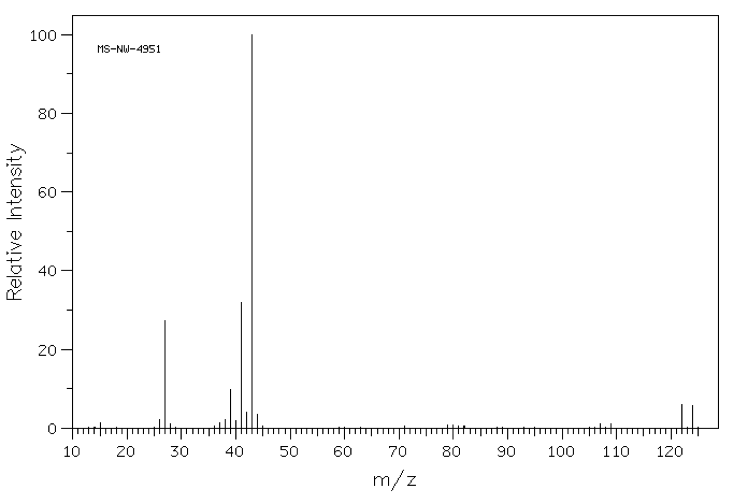
Chlorine
Oxygen
Sulfur
Phosphorus
Bromine
Bromine
The basic principle being tested here is that mass spectra show the exact mass of each molecular fragment (originally derived from the sample) detected in the instrument. This means that, while we often think of the atomic mass of elements as a weighted average of the masses of the respective isotopes of an element, mass spectrometry reveals the isotopic variances in these fragments.
The correct answer is bromine, as indicated by the "doublet" feature centered at 121 m/z units. This doublet arises due to the fact that 79Br and 81Br each occur naturally in equal abundance; thus half of the molecules in a monobrominated sample will contain a 79Br atom, giving rise to the peak at the lower m/z value, and the other half, containing an 81Br, will give rise to a peak two m/z units higher.
Another important elemental mass spectrum signature to remember is that of chlorine, which occurs as 35Cl (75% abundance) and 37Cl( 25% abundance). A mass spectrum of a molecule containing chlorine will give a similar doublet, but the lower m/z value peak will be three times as large as the peak two mass units higher.
Example Question #41 : Laboratory Practices
A reaction is performed that produces a mixture of cis- and trans-2-butene. What is the best analytical method to quantify the ratio of these isomers (shown)?
Gas chromatography
Mass spectrometry
Infrared spectroscopy
Thin layer chromatography
H NMR
Gas chromatography
The two alkenes have a boiling point difference of 
Thin layer chromatography is rarely used for quantitative analysis and the alkenes are much too volatile to be tested with this method. Mass spectrometry is a excellent method, however, both of these compounds have identical molecular masses and would involve complex analysis of fragments, if possible at all. Lastly, infrared spectroscopy is used to identify functional groups (qualitative) and is rarely used for quantitative analysis.
Certified Tutor
All Organic Chemistry Resources





