All Organic Chemistry Resources
Example Questions
Example Question #1 : Other Intermolecular Forces
Rank the following compounds in terms by increasing boiling point, starting with the lowest boiling point first.
I. 1-pentanol
II. n-pentane
III. 2,2-dimethylpropane
IV. (R)-4-hydroxypentanoic acid.
II < III < IV < I
III < II < I < IV
III < II < IV < I
IV < I < II < III
II < III < I < IV
III < II < I < IV
Boiling point is highly dependent on the intermolecular forces of a compound. Compounds with stronger intermolecular forces, larger masses, and less branching will have higher boiling points.
Compounds II and III only exhibit intermolecular London dispersion forces, so they would be the two lowest boiling compounds (weakest intermolecular forces). Because compound III has more branching, these London dispersion forces would be weaker, resulting in a lower boiling point than compound II.
III < II
Compounds I and IV would be higher boiling point compounds because of additional hydrogen bonding (strong intermolecular forces). Compound IV would be the highest boiling because the hydroxy group and carboxylic acid group could BOTH participate in intermolecular hydrogen bonding. In addition, compound IV is more polar (more polarized carbon-oxygen bonds), resulting in greater dipole-dipole attraction as well.
III < II < I < IV
Example Question #1 : Intermolecular Forces And Stability
Which of the following statements is true of alkynes?
The triple bond of an alkyne consists of three pi-bonds
Terminal alkynes are less acidic than internal alkynes
Internal alkynes are more stable than terminal alkynes
Alkynes are very soluble in water
Terminal alkynes are stronger compounds than internal alkynes
Internal alkynes are more stable than terminal alkynes
The answer is "Internal alkynes are more stable than terminal alkynes" as it is the only true statement in regards to alkynes. Internal alkynes are more stable because they have a better conjugated system than terminal alkynes. A conjugated system is a system of a single bond, then a multiple bond, then a single bond, and so on. A conjugated system will always be more stable than an unconjugated system. It is evident that the internal alkyne follows the conjugated system and the terminal alkyne does not based on the picture below.

Example Question #1 : Intermolecular Forces And Stability
Which of the following compounds will be the most volatile (have the lowest boiling point)?
![]()
I
V
IV
II
III
III
When comparing relative volatilities of compounds, you must consider the molecular weight of a compound, as well as the intermolecular attractive forces between the identical molecules found in a sample of the compound in question.
We can eliminate choices I, II and V. These compounds have functional groups that feature polarized X-H bonds, allowing molecules in a sample of these compounds to participate in hydrogen bonding. Hydrogen bonding is a strong attractive force, and thus more energy would have to be put into a sample to vaporize it (boil a liquid sample). In other words, the hydrogen bonds will raise the boiling point and lower the volatility of these compounds.
Answer choice IV, which features an alkyl bromide, may also be eliminated for two reasons. First, as bromine is much heavier than carbon, molecule IV will be much heavier than III, and will thus require much more energy to transition into the gaseous state. Secondly, as bromine is fairly electronegative, the molecule will feature a dipole in the carbon-bromine bond, and thus a sample of IV will experience dipole-dipole attractive interactions. As described above, attractive intermolecular interactions require more energy to overcome in order for a sample to undergo a liquid-gas phase change. Thus, molecule IV is less volatile than molecule III, the correct answer.
Example Question #1 : Intermolecular Forces And Stability
Which of the following will have the highest vapor pressure?
Octane
Ethanol
Pentane
Acetic acid
Pentane
The compound with the highest vapor pressure will have the weakest intermolecular forces. Octane and pentane have only London dispersion forces; ethanol and acetic acid have hydrogen bonding. Hydrogen bonding is much stronger than London dispersion forces. Because octane is larger than pentane, it will have more London dispersion forces, thus pentane has the weakest intermolecular forces.
Example Question #1 : Intermolecular Forces And Stability
Which of the following molecules has the highest boiling point?

Example Question #3 : Intermolecular Forces And Stability
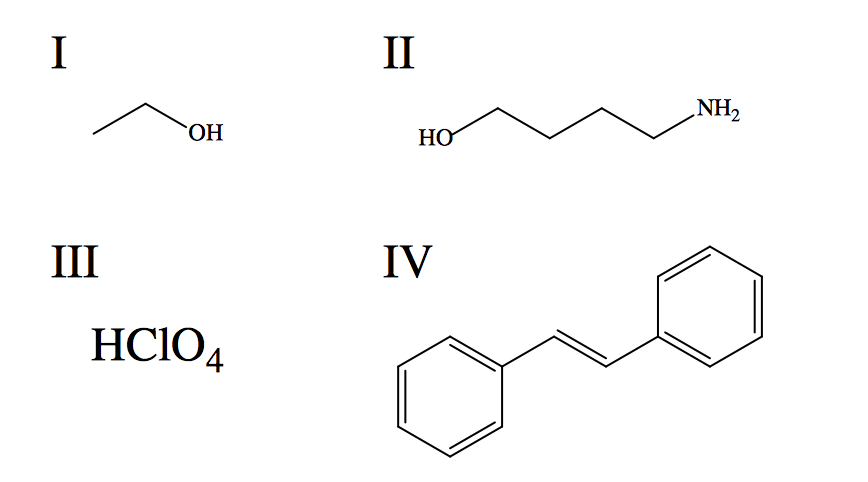
Rank the given species in terms of increasing aqueous solubility.
IV, II, I, III
II, IV, III, I
I, II, III, IV
IV, I, II, III
II, IV, I, III
IV, II, I, III
"Like dissolves like" is a good guiding principle to keep in mind in dealing with solubility trends. In other words, polar solvents will more easily dissolve polar solutes than nonpolar; and vice versa. Water is a polar solute that forms strong hydrogen bonds (intramolecular and intermolecular), which are energetically favorable interactions. Solutes that are also capable of hydrogen bonding are readily dissolved in water since they do not significantly disrupt the network of intramolecular hydrogen bonds. In order to predict the solubilities of the given compounds, it is useful to define the primary intermolecular forces each experiences when introduced to water. I: Hydrogen bonding dominates interaction between methanol and water (the two are miscible). II: Hydrogen bonding is present, but solubility is reduced by the presence of a multi-carbon chain, which adds significant nonpolar character to the structure. III: Perchloric acid is a strong acid (stronger than nitric acid and sulfuric acid), meaning it completely dissociates in water, forming very strong ion-dipole interactions with water. Assessment of the given interactions leads to the correct trend of increasing solubility: IV, II, I, III.
Example Question #2 : Intermolecular Forces And Stability
Which of the following molecules has the lowest vapor pressure?
The molecule with the lowest vapor pressure is the molecule with the strongest intermolecular forces. All of these molecules except pentane have the capability to hydrogen bond. However, 
Example Question #1 : Help With Intermolecular Forces
Of the following intermolecular forces, which force would typically provide a pure compound with the highest possible boiling point?
Network covalent forces
Hydrogen bonding
Dipole-dipole interractions
Ionic bonding
Network covalent forces
At first glance, we would be eager to jump to ionic bonding as the correct answer, as ionic bonding provides for very high boiling points. The correct answer, however, is a rare type of intermolecular force called network covalent bonding. Network covalent bonding is typically seen in diamond and quartz, and is a stronger intermolecular force than ionic bonding. Hydrogen bonding is the next strongest intermolecular force and also increases the boiling points of pure substances.
Example Question #1 : Intermolecular Forces And Stability
Which of the following has the lowest boiling point?
Isobutane
Pentane
Isopentane
Butane
Isobutane
In general, increased intermolecular interraction and higher magnitude of intermolecular forces lead to an increase in a molecule's boiling point. Inversely, decreased intermolecular interraction and lower magnitude of intermolecular forces lead to a decrease in a molecule's boiling point.
In this case, the only intermolecular force exhibited by any of these molecules are London dispersion forces. The magnitude of London dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area). Therefore, the shortest, most branched molecule in this problem will have the lowest boiling point. The correct answer is isobutane, a four membered, branched hydrocarbon.
Example Question #231 : Organic Concepts
Which of the following molecules has the lowest boiling point?
Pentane
2,3-dimethylbutane
Hexane
2-methylpentane
2,3-dimethylbutane
When discussing boiling points of hydrocarbons, it is important to remember that branching decreases a molecule's boiling point. We can first eliminate hexane and pentane as our answers, as neither are branched. From here, we can come upon 2,3-dimethylbutane as our answer because it is more branched than 2-methylpentane. Also important when ranking hydrocarbons in terms of boiling point is the number of carbons - more carbons means a higher boiling point.
All Organic Chemistry Resources












