All Organic Chemistry Resources
Example Questions
Example Question #1 : Specific Reactions And Named Reactions
When the following reaction is carried out, what kind of product is formed:

Note: When an organic reaction employs heat, it is often shown as a delta over the reaction arrow.
A tricyclic system with two five-membered rings and a six-membered ring
A bicyclic system with a five-membered ring and a six-membered ring
A tricyclic system with a five-membered ring and two six-membered rings
A bicyclic system with two six-membered rings
A tricyclic system with a four-membered ring, a five-membered ring, and a six-membered ring
A tricyclic system with a five-membered ring and two six-membered rings
This is a Diels-Alder reaction; these reactions happen between a nucleophilic diene, shown in blue below, and an electrophilic dienophile, in green. Diels-Alder reactions install a set of bonds that connect each external carbon of the diene system to an alkene carbon in the dienophile system to create a new six-membered ring. All remaining structure of the two reactants are retained, including the six- and five-membered rings below. The red bonds are the newly installed bonds.

Example Question #1 : Specific Reactions And Named Reactions
What is the product of the reaction between 1,3-dibutene and bromoethene?
None of these
3-bromocyclohexene
4-bromocyclohexene
3-bromocyclopentene
No reaction occurs
4-bromocyclohexene
The electrons from one of the double bonds on the 1,3-dibutene create a new single bond. The other new single bond is created from the electrons in the double bond of the other reactant. These two new single bonds join the reactants to create a cyclic product.
The electrons from the other double bond in the 1,3-dibutene move between the carbon 2 and 3. Thus, the final product is a 6-carbon cycloalkene with a halogen substituent.
Example Question #1 : Specific Reactions And Named Reactions
What reagent(s) is/are needed to drive the given reaction?
This is a standard Diels-Alder reaction. Diels-Alder reactions are driven solely by adding heat to the reagents. By looking at the reagents and the product, we can tell that this is a Diels-Alder reaction. For Diels-Alder, we need a cis-diene and an alkene as reactants. When these reactants are stimulated by heat, they form a cyclohexene product.
Example Question #1 : Specific Reactions And Named Reactions

What is the product of the given reaction?






This is a classic Diels-Alder reaction and it consists of a diene (cyclopentadiene) and a dienophile (ethene). The bicyclic structure forms if the electrons are moved in a circular fashion.
Example Question #2 : Help With Diels Alder Reactions
What is the product of the given reaction?

II
III
V
I
IV
II
Diels-Alder reactions create cyclohexene rings (eliminate III, IV, and V), and starting dienophile is trans (E conformation), so product is E (Eliminate I).
Example Question #1 : Hydrocarbon Reactions
What reaction forms a substituted cyclohexene system?
Gabriel synthesis
Wittig reaction
Hoffmann elimination
Diels-Alder reaction
Diels-Alder reaction
The Diels-Alder reaction converts a conjugated diene and a substituted alkene into a six-membered ring containing cyclohexene (a substituted cyclohexene system). In Hoffmann elimination, tetra-alkyl ammonium salts undergo elimination to form the least substituted alkene. The Wittig reaction uses phosphorus ylides, aldehydes, or ketones to form an alkene and a triphenylphosphine oxide. Lastly, Gabriel synthesis forms primary amines via the reaction of a phthalimide with an alkyl halide, followed by cleavage with hydrazine.
Example Question #2 : Hydrocarbon Reactions
Which substrate, when subjected to ozonolysis followed by treatment with dimethyl sulfide, would give only one hydrocarbon product?
3-octene
1,7-octadiene
2-octene
1,3-octadiene
4-octene
4-octene
Ozonolysis is essentially used to cleave a compound at the location of a double bond. For the result to be a single product, the cleavage must occur at a point of symmetry.
4-octene, a symmetrical alkene, would give two equivalents of butanal upon ozonolysis. All of the other compounds are unsymmetrical and would give at least two different non-identical products.
Example Question #1 : Help With Ozonolysis
Ozonolysis of an alkyne results in the products 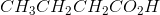

None of these
2,2-dimethyl-3-octyne
5,5-dimethyl-3-heptyne
3,3-dimethyl-2-heptyne
2,2-dimethyl-3-heptyne
2,2-dimethyl-3-heptyne
Ozonolysis of the alkyne breaks the alkyne at the triple bond. The carbons that were in the triple bond become the carbons of either a ketone or a carboxylic acid depending on if they are terminal or not.
Working backwards, one can take the products and remove their oxygens. Then, the carbons from which the oxygens were removed should be triple bonded. The initial compound has seven carbons in its longest chain. Counting from the side closest to the bond, C2 has two methyl groups bound to it. The triple bond runs across C3 and C4. Thus the answer is 2,2-dimethyl-3-heptyne.
Example Question #2 : Specific Reactions And Named Reactions
An organic chemist reacts one mole of 4-octene with excess 
Two moles of butal
None of these
Octane
Two moles of octanol
Two moles of butal
These are standard conditions for an ozonolysis reaction. For an ozonolysis reaction to take place, all we need is an alkene and ozone. The ozone essentially cuts the alkene in half and adds a carbonyl group to each half of the carbon chain.
Example Question #1 : Specific Reactions And Named Reactions
Cyclohexene undergoes hydrobromination.
Which of these is a possible product?
Cis 1,2-dibromocyclohexane
All of these
None of these
Trans 1,2-dibromocyclohexane
Bromocyclohexane
Bromocyclohexane
Only bromocyclohexane is created because there is only one bromine group that bonds with one of the carbons, while the other carbon is bonded with hydrogen group.
Certified Tutor
All Organic Chemistry Resources













