All SAT II Chemistry Resources
Example Questions
Example Question #1 : Molecules
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?
Possible Answers:




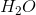
Correct answer:

Explanation:

Bhavi
Certified Tutor
Certified Tutor
Touro College, Master of Science, Biological and Physical Sciences. Touro College, Doctor of Medicine, Osteopathic Medicine (...
Brianna
Certified Tutor
Certified Tutor
Fairfield University, Bachelor of Science, Biology, General. National University of Ireland at Galway, Master of Science, Neu...
All SAT II Chemistry Resources
Popular Subjects
Algebra Tutors in Dallas Fort Worth, Physics Tutors in New York City, Spanish Tutors in Houston, Reading Tutors in Denver, Physics Tutors in San Francisco-Bay Area, LSAT Tutors in Seattle, Computer Science Tutors in Philadelphia, SSAT Tutors in Washington DC, Reading Tutors in Atlanta, Math Tutors in New York City
Popular Courses & Classes
ISEE Courses & Classes in Los Angeles, LSAT Courses & Classes in New York City, SAT Courses & Classes in New York City, ACT Courses & Classes in Houston, MCAT Courses & Classes in Miami, GRE Courses & Classes in Houston, SSAT Courses & Classes in Philadelphia, ACT Courses & Classes in San Francisco-Bay Area, GMAT Courses & Classes in Los Angeles, LSAT Courses & Classes in Miami
Popular Test Prep
ISEE Test Prep in Chicago, ACT Test Prep in Houston, LSAT Test Prep in Washington DC, GRE Test Prep in Los Angeles, SAT Test Prep in Washington DC, LSAT Test Prep in Miami, SAT Test Prep in New York City, GMAT Test Prep in Washington DC, MCAT Test Prep in Seattle, LSAT Test Prep in Los Angeles




