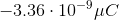All AP Physics 2 Resources
Example Questions
Example Question #801 : Ap Physics 2
You have 

Because we're talking about electrons, the answer must be negative. The way to solve this is to find how many electrons are in 1.5 kg of water. First, we need to convert kilograms of water into grams of water:
Then, we can use the provided molar mass of water to calculate the number of moles of water in 1.5kg of water:
Once we know how many moles of water we have, we can use Avogadro's number (
Once we know how many molecules of water we have, we can multiply by 10 to figure out how many electrons those molecules represent, since we are told that each water molecule has 10 electrons.
Finally, we can multiply by the provided charge of an electron to calculate the charge of those electrons.
Example Question #12 : Measurements
You have a neutral balloon. If you were to add 21,000 electrons to it, what would its net charge be?

None of the other answers is correct
The elemental charge is the magnitude of charge, in Coulombs, that each electron or proton has. Because electrons have a negative charge, don't forget to add a negative sign into the equation.
When you convert the answer to microcoulombs, the answer is 
Example Question #1 : Charge Distribution
Which of the following best represents the charge of an electron?
The equation for the quantity of charge is:
where 


Rewrite the equation.
One coulomb, 

Substitute these two values into the formula.
This number represents the electron's fundamental charge.
Example Question #2 : Charge Distribution
Imagine you have a neutral balloon. If you remove 16,000 electrons from it, what is the net charge on the balloon?
Because this is a neutral balloon, the net charge is equal to the charge the was removed, but opposite in sign. There were 16,000 electrons removed, each of which has a charge of 
To answer the question, we must remember that if that much charge was removed from the balloon, the balloon will now be negative.
Example Question #1 : Charge Distribution
A hollow metal sphere of radius 

Surface area of sphere:
Plug in values:
All AP Physics 2 Resources