All AP Physics 2 Resources
Example Questions
Example Question #9 : Atomic And Nuclear Physics
By what process is 


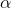

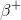
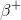
To answer this question, we'll need to consult the periodic table. From the table, we know that magnesium's atomic number (the number of protons it contains in its nucleus) is 12, and sodium's is 11. We also need to realize that the mass number for each (the number of protons plus neutrons contained in the nucleus) is the same. Since the mass numbers are the same but the atomic numbers differ by one, then we can infer that a neutron is undergoing a decay into a proton and a so called positron, 
Furthermore, it cannot be alpha decay, because in this process an alpha nucleus is released and the reactant's mass number and atomic number would both change. It also cannot be gamma decay, because in this process there is no change in atomic or mass numbers. Finally, it cannot be electron capture because in this process, an electron combines with a proton to generate a neutron. Thus, the mass number would not change, but the atomic number would increase by one. But in the question stem, we know the atomic number is decreasing by one rather than increasing.
Example Question #1 : Atomic And Nuclear Physics
Which of the following particles has a charge that is fractions of an electron charge?
Quark
Tachyon
Tau
Hadron
Graviton
Quark
The correct answer is quarks. Quarks usually have charges of 
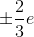

Example Question #1 : Subatomic Particles
Which of the following subatomic particles has the highest charge to mass ratio?
Antiproton
Gluon
Neutron
Proton
Electron
Electron
Neutrons and gluons both have no charge at all, so they can be ignored. The proton and the antiproton have the same mass but opposite charges, and so have the same ration of charge:mass. However, the electron has equal charge to both the proton and the antiproton, and has a ridiculously small mass comparatively. The mass of a proton/antiproton is 
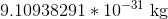
All AP Physics 2 Resources





