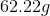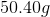All AP Physics 2 Resources
Example Questions
Example Question #1 : Laws Of Thermodynamics
Calculate the entropy change when 2kg of ice melts.
Since we know the heat of fusion of water on a per mass basis, we can calculate the heat required to melt 2kg of ice:
Ice melts at a constant temperature of 
Now, use the equation for entropy:
Example Question #2 : Thermodynamics
If the isothermal expansion of a gas inside a container absorbs 25 kJ of heat energy, how much work is done by the gas in this process?
No work is done
Recall that in an isothermal process, the change in internal energy of the has is 0. Thus, we can make use of the following equation to calculate the work done.
Since the change in internal energy is 0, we can rewrite the equation as:
In other words, in order to maintain a constant internal energy of the system, the gas must do the same amount of work on its surrounding as the amount of energy it absorbs from the surroundings in the form of heat.
Example Question #3 : Laws Of Thermodynamics
Which of the following is not one of the laws of thermodynamics?
Energy can only be converted from one form to another; it cannot be created
Energy and mass are interconvertible
All spontaneous processes must lead to an increase of entropy in the universe
At a temperature of absolute zero, all motion within a system ceases
If two systems are in each in thermal equilibrium, respectively, with a third system, then they must be in thermal equilibrium with each other
Energy and mass are interconvertible
In order to find out which of the answer choices is not one of the laws of thermodynamics, we'll need to consider each one.
The zeroth law of thermodynamics says that if two systems are in each in thermal equilibrium, respectively, with a third system, then they must be in thermal equilibrium with each other.
The first law of thermodynamics says that energy can only be converted from one form to another and cannot be created.
The second law of thermodynamics says that all spontaneous processes must lead to an increase of entropy in the universe.
The third law of thermodynamics says that at a temperature of absolute zero, all motion within a system ceases.
The only other answer choice left states that energy and mass are interconvertible. Although this is a true statement, as is stated by Einstein's famous equation 
Example Question #4 : Laws Of Thermodynamics




We can use the 1st law of thermodynamics to solve this problem:
We are given the heat applied to the system, so we need to calculate the work done by the system. Since we were told that this is isobaric expansion, we can use the following expression for work:
Substituting this in, we get:
Plugging in values, we get:
Example Question #5 : Laws Of Thermodynamics




We will begin with the 1st law of thermodynamics:
We know that there is no change in internal energy, so we can say:
Also, the problem statement tells us that this is isothermal expansion, we can use the following expression for work:
Plugging this into our expression, we get:
Rearranging for the change in volume, we get:
We have all of these values, so time to plug and chug:
Example Question #6 : Laws Of Thermodynamics
In an isothermal process, you are told that heat is being added to the system. Which of the following is not true?
Work is being done on the system.
The gas is expanding.
Work is being done by the system.
The pressure of the gas is decreasing.
The average kinetic energy of the particles is remaining constant.
Work is being done on the system.
In an isothermal process, there are two possibilities: the gas is expanding or the gas is being compressed. If the gas is expanding, the gas is doing work and therefore needs heat in order to remain at constant temperature (which is the definition of an isothermal process). If the gas is compressed, then work is being done on the gas and heat must be emitted in order for the temperature to remain constant. We're told heat is added, so the gas must be expanding. This means the volume is increasing and work is being done by the gas. Therefore, those two choices are not the answer. The pressure must decrease, which can be seen using the formula 
Both of these conceptual methods of figuring out if heat must be added or released can be mathematically computed using the formula 



Example Question #7 : Laws Of Thermodynamics
A piston alters the gas held in a sealed chamber. If 


Here, we need to use the first law of thermodynamics:
Since the heat is added to the system, the sign is positive. Moreover, the work can be calculated using:
Now, we can simply add these two parts to get the change in internal energy.
Example Question #1 : Heat Transfer And Thermal Equilibrium



There is not enough information to determine the rod's mass
The relevant equation for this problem is called the specific heat capacity equation:
In this equation, 




To find the mass of the rod when given the energy, specific heat, and change in temperature, we can rearrange the equation to this:
Now, we can plug in our numbers and solve:
Example Question #1 : Thermodynamics
Which of the following best defines the zeroth law of thermodynamics in variable form?
The zeroth law of thermodynamics states that if an object X is in thermal equilibrium with object Y, and object Y also in thermal equilibrium with object Z, then object X must also be in thermal equilibrium with object Z.




The best choice is:
Example Question #1 : Heat Transfer And Thermal Equilibrium
You have 


There's not enough information to determine the temperature change
The equation for temperature change given applied heat is






Rearranging to find the change in temperature:
Now, we can plug in our numbers.
Therefore, the water changed by about 
Certified Tutor
All AP Physics 2 Resources