All College Chemistry Resources
Example Questions
Example Question #41 : Solutions, States Of Matter, And Thermochemistry
One flask is at STP and another is at 
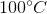
Possible Answers:
Correct answer:
Explanation:
The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Example Question #1 : Non Ideal Gas Behavior
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.

Possible Answers:
Correct answer:
Explanation:
Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
All College Chemistry Resources
Popular Subjects
Biology Tutors in Phoenix, Physics Tutors in Atlanta, Reading Tutors in Atlanta, Calculus Tutors in Boston, ACT Tutors in Atlanta, Algebra Tutors in Chicago, Calculus Tutors in Phoenix, English Tutors in Phoenix, Statistics Tutors in Washington DC, LSAT Tutors in Philadelphia
Popular Courses & Classes
GRE Courses & Classes in Boston, Spanish Courses & Classes in Chicago, Spanish Courses & Classes in Los Angeles, SAT Courses & Classes in Los Angeles, ACT Courses & Classes in San Francisco-Bay Area, SAT Courses & Classes in Seattle, ISEE Courses & Classes in Atlanta, GRE Courses & Classes in Dallas Fort Worth, SAT Courses & Classes in Boston, SSAT Courses & Classes in Boston
Popular Test Prep
MCAT Test Prep in New York City, GRE Test Prep in Seattle, GMAT Test Prep in New York City, ACT Test Prep in Boston, LSAT Test Prep in Denver, LSAT Test Prep in Chicago, GRE Test Prep in Dallas Fort Worth, LSAT Test Prep in Houston, ISEE Test Prep in San Francisco-Bay Area, GMAT Test Prep in Boston


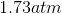
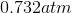

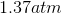


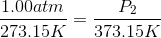



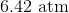



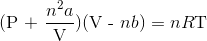
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)





