All GED Science Resources
Example Questions
Example Question #11 : Ged Science
A student is asked to make 

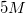
Possible Answers:
Correct answer:
Explanation:
Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
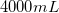
All GED Science Resources
Popular Subjects
ISEE Tutors in Houston, SSAT Tutors in Phoenix, Statistics Tutors in Chicago, MCAT Tutors in Miami, Statistics Tutors in Philadelphia, Calculus Tutors in San Diego, LSAT Tutors in San Diego, Statistics Tutors in San Francisco-Bay Area, Physics Tutors in Boston, Computer Science Tutors in Chicago
Popular Courses & Classes
SSAT Courses & Classes in Los Angeles, ACT Courses & Classes in San Francisco-Bay Area, SAT Courses & Classes in Washington DC, SSAT Courses & Classes in San Diego, MCAT Courses & Classes in Houston, ACT Courses & Classes in Phoenix, MCAT Courses & Classes in San Diego, ISEE Courses & Classes in Chicago, LSAT Courses & Classes in Phoenix, MCAT Courses & Classes in Chicago
Popular Test Prep
ACT Test Prep in Atlanta, LSAT Test Prep in Houston, SAT Test Prep in Philadelphia, MCAT Test Prep in Washington DC, SSAT Test Prep in Atlanta, GMAT Test Prep in Houston, ISEE Test Prep in Denver, MCAT Test Prep in Philadelphia, MCAT Test Prep in San Francisco-Bay Area, ISEE Test Prep in Houston


















