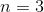All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Other Reactions
Which of these methods can be used to synthesize a secondary alcohol?
All of these answer choices are correct
Reacting a ketone with lithium aluminum hydride, then adding water
Reacting propene with water and acid
Adding a Grignard reagent to an aldehyde, then reacting with acid
All of these answer choices are correct
All of these methods can successfully synthesize secondary alcohols.
Lithium aluminum hydride and sodium borohydride are strong and weaker reducing agents, respectively. Both are able to reduce ketones to secondary alcohols.
Adding water and acid to an alkene (such as propene) results in Markovnikov addition of a hydroxyl group, also creating a secondary alcohol.
Grignard reagents (organometallic halides) add to the carbonyl carbon of an aldehyde, adding an alkane group and forming an alcohol product.
Example Question #22 : Functional Group Reactions
Ephedrine (shown below) contains what type of amine?

Primary
Quaternary
Neutral
Secondary
Tertiary
Secondary
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Example Question #21 : Functional Group Reactions
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
A primary halide
A tertiary carbocation
A good nucleophile
A polar aprotic solvent
A tertiary carbocation
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
Example Question #2 : Reaction Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Using the product of reaction 2, a scientist adds bromine gas to the reaction chamber. After the bromine and the alkene react, he finds that his product consists entirely of single bonds, with two bromine atoms on the carbon chain. What kind of reaction most likely took place?
Substitution reaction
Oxymercuration/demercuration reaction
Elimination reaction
Halogenation reaction
Addition reaction
Addition reaction
The addition of bromine gas (
Halogenation reactions refer to reactions between a halogen and an alkane, while addition reactions occur between a halogen and an alkene (such as the product in reaction 2).
Example Question #1451 : Mcat Biological Sciences
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
II and III
I and II
I, only
I and III
III, only
I and III
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
Example Question #1452 : Mcat Biological Sciences
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Reaction rate in this reaction is not determined by concentration
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Example Question #23 : Functional Group Reactions
Which of the following amino acids can participate in the formation of a disulfide bridge?
Aspartic acid
Tyrosine
Cysteine
Phenylalanine
Alanine
Cysteine
Cysteine's R-group contains a sulfhydryl group (-SH), which can participate in the formation of a disulfide bridge in a protein's tertiary and/or quaternary structure. Cysteine is the only amino acid to contain a sulfur atom.
Example Question #2 : Hydrocarbon Reactions
Which substrate, when subjected to ozonolysis followed by treatment with dimethyl sulfide, would give only one hydrocarbon product?
3-octene
1,7-octadiene
2-octene
1,3-octadiene
4-octene
4-octene
Ozonolysis is essentially used to cleave a compound at the location of a double bond. For the result to be a single product, the cleavage must occur at a point of symmetry.
4-octene, a symmetrical alkene, would give two equivalents of butanal upon ozonolysis. All of the other compounds are unsymmetrical and would give at least two different non-identical products.
Example Question #2 : Help With Alkene Synthesis

What is the value of 
Huckel's rule states that an aromatic compound must have 
If 4n+2=18, then n=4.
Certified Tutor
All GRE Subject Test: Chemistry Resources