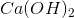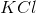All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Oxidation Reduction Reaction
Which substance is used as a reducing agent?
Possible Answers:
Correct answer:
Explanation:
A reducing agent is a substance that readily donates an electron to another substance. They consist primarily of elements with low electronegativities such as hydrogen. Therefore, the answer is 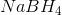
Nate
Certified Tutor
Certified Tutor
University of Wisconsin-Oshkosh, non degree, Prepharmacy. University of Wisconsin-Madison, Pharmaceutical Chemist, Pharmacy.
Beatrice
Certified Tutor
Certified Tutor
Michigan Technological University, Bachelor of Science, Biomedical Engineering. Michigan Technological University, Bachelor o...
All GRE Subject Test: Chemistry Resources
Popular Subjects
LSAT Tutors in Washington DC, GMAT Tutors in Miami, Computer Science Tutors in New York City, French Tutors in Boston, Computer Science Tutors in Phoenix, ISEE Tutors in Phoenix, Biology Tutors in Chicago, LSAT Tutors in Seattle, Algebra Tutors in Denver, ISEE Tutors in Dallas Fort Worth
Popular Courses & Classes
ACT Courses & Classes in Chicago, LSAT Courses & Classes in Atlanta, SAT Courses & Classes in Dallas Fort Worth, Spanish Courses & Classes in Seattle, SAT Courses & Classes in Philadelphia, LSAT Courses & Classes in Phoenix, SAT Courses & Classes in Houston, MCAT Courses & Classes in San Diego, ACT Courses & Classes in Miami, SSAT Courses & Classes in Dallas Fort Worth
Popular Test Prep
ACT Test Prep in Chicago, MCAT Test Prep in Chicago, LSAT Test Prep in Houston, ISEE Test Prep in Miami, SSAT Test Prep in San Diego, ACT Test Prep in Phoenix, MCAT Test Prep in Dallas Fort Worth, GMAT Test Prep in San Diego, ISEE Test Prep in Washington DC, ISEE Test Prep in San Diego