All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #221 : Gre Subject Test: Chemistry
Which law of thermodynamics states that a crystal's entropy at a temperature of absolute zero is equal to 0?
Second law
First law
Third law
Zeroth law
Third law
There are four laws of thermodynamics:
Zeroth law of thermodynamics: If two independent systems are each in thermodynamic equilibrium with a thrid independent system, then the first two systems must be in thermodyanmic equilibrium with each other.
First law of thermodynamics: Energy is neither created nor destroyed.
Second law of thermodynamics: The entropy of the universe increases following every reaction.
Third law of termodynamics: At a temperature of 0 Kelvin (absolute zero), the entropy of the crystal is 0.
Example Question #1 : Thermodynamics And Phases
Propane gas combusts according the following chemical equation:
Given the following standard enthalpy values, what is the enthalpy of the reaction for the combustion of one mole of propane?
Given the molar enthalpy values for reactants and products, we can solve for the enthalpy of the reaction using the equation:
Keep in mind that the molar enthalpy values must be multiplied by the coefficients that are present in the balanced reaction:
Oxygen is omitted because its enthalpy value is zero.
Example Question #1 : General Thermodynamics
Which is not characteristic of an endothermic reaction?
Releasing of energy to surroundings
A chemical reaction that feels cold
The net change of enthalpy is positive
Breaking of a chemical bond
Absorption of energy from surroundings
Releasing of energy to surroundings
An endothermic reaction absorbs energy in the form of heat. An endothermic reaction involves breaking of chemical bonds because it involves the absorption of energy. Endothermic reactions tend to feel cold because it is taking heat away from your skin. Since endothermic reactions involve absorbing energy, often in the form of heat, the change in enthalpy is positive.
Therefore, the answer is "releasing of energy to surroundings" which is not a characteristic of an endothermic process. Instead, it is a characteristic of an exothermic reaction which is the direct opposite of an endothermic reaction.
Example Question #51 : Physical Chemistry
Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.
Example Question #2 : General Thermodynamics
Which of the following reactions has a positive value for change in entropy 
Water freezing into ice
Water evaporates into water vapor
A salt precipitates out of a saturated solution
Water evaporates into water vapor
Entropy is a measure of the disorder of a system. In other words, when the components of a system become more uniform and spread out, the entropy of the system has increased. For example, when a liquid becomes gaseous, the molecules separate from one another, increasing the disorder of the system. As a result, the evaporation of water results in an increase in entropy.
All other options result in a more ordered system, which decreases entropy.
Example Question #4 : Physical Chemistry
At 


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:
Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:
Example Question #3 : Physical Chemistry
Which of the following options indicates that a chemical reaction is unfavorable?
All of these
The sign of 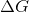



Example Question #1 : Colligative Properties
What is the freezing point of a 2M solution of 
First, we need to calculate the molality because that is what we use in our equation for freezing point depression. We can get that from the molarity without knowing exactly how many liters or grams we have. We just have to know what we have one mole per liter. The weight of water is one kilogram per liter, so this allows us to make this conversion.
The molality is 2m. The van't Hoff factor is 3, as we get one calcium ion and two chloride ions per molecule during dissociation.
We can now plug the values into the equation for freezing point depression.
This gives us our depression of 


Example Question #5 : Physical Chemistry
How much sodium chloride has been added to four liters of water if the freezing point of the solution is 
Sodium chloride has a molar mass of 
We can determine how much sodium chloride was added to the water using the freezing point depression equation.
The normal freezing point of wtaer is 0 degrees Celsius, so we know that the temperature of the solution has changed by 2.5 degrees. Since sodium chloride will generate two ions per molecule in solution, the van't Hoff factor will be 2. Based on the density of water, we can determine that 4 liters of water weighs 4 kilograms.
Example Question #6 : Physical Chemistry
Two moles of sodium chloride (NaCl) are added to 1kg of a mystery solvent. The addition of the NaCl caused an increase of 6K to the solvent's boiling point.
Based on this information, what is the boiling constant for the solvent?
In order to solve this problem, we can use the boiling point elevation equation: 
We know the temperature change, we can compute molality from the given information, and we know the van't Hoff factor (expected to be 2 in this scenario due to NaCl becoming 2 ions in solution). We can calculate the boiling point constant for the solvent.
Certified Tutor
All GRE Subject Test: Chemistry Resources








































































