All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Thermodynamics And Phases
Which law of thermodynamics states that a crystal's entropy at a temperature of absolute zero is equal to 0?
Third law
Second law
Zeroth law
First law
Third law
There are four laws of thermodynamics:
Zeroth law of thermodynamics: If two independent systems are each in thermodynamic equilibrium with a thrid independent system, then the first two systems must be in thermodyanmic equilibrium with each other.
First law of thermodynamics: Energy is neither created nor destroyed.
Second law of thermodynamics: The entropy of the universe increases following every reaction.
Third law of termodynamics: At a temperature of 0 Kelvin (absolute zero), the entropy of the crystal is 0.
Example Question #2 : Thermodynamics And Phases
Propane gas combusts according the following chemical equation:
Given the following standard enthalpy values, what is the enthalpy of the reaction for the combustion of one mole of propane?
Given the molar enthalpy values for reactants and products, we can solve for the enthalpy of the reaction using the equation:
Keep in mind that the molar enthalpy values must be multiplied by the coefficients that are present in the balanced reaction:
Oxygen is omitted because its enthalpy value is zero.
Example Question #1 : Physical Chemistry
Which is not characteristic of an endothermic reaction?
A chemical reaction that feels cold
Absorption of energy from surroundings
Releasing of energy to surroundings
The net change of enthalpy is positive
Breaking of a chemical bond
Releasing of energy to surroundings
An endothermic reaction absorbs energy in the form of heat. An endothermic reaction involves breaking of chemical bonds because it involves the absorption of energy. Endothermic reactions tend to feel cold because it is taking heat away from your skin. Since endothermic reactions involve absorbing energy, often in the form of heat, the change in enthalpy is positive.
Therefore, the answer is "releasing of energy to surroundings" which is not a characteristic of an endothermic process. Instead, it is a characteristic of an exothermic reaction which is the direct opposite of an endothermic reaction.
Example Question #1 : Physical Chemistry
Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.
Example Question #4 : Thermodynamics And Phases
Which of the following reactions has a positive value for change in entropy 
Water freezing into ice
Water evaporates into water vapor
A salt precipitates out of a saturated solution
Water evaporates into water vapor
Entropy is a measure of the disorder of a system. In other words, when the components of a system become more uniform and spread out, the entropy of the system has increased. For example, when a liquid becomes gaseous, the molecules separate from one another, increasing the disorder of the system. As a result, the evaporation of water results in an increase in entropy.
All other options result in a more ordered system, which decreases entropy.
Example Question #2 : Physical Chemistry
At 


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:
Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:
Example Question #3 : Physical Chemistry
Which of the following options indicates that a chemical reaction is unfavorable?
All of these
The sign of 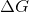



Example Question #352 : Gre Subject Test: Chemistry
Use the following values for water as needed.
If burning wood releases 



There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from 


2. Boiling the water at a constant temperature of 

To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.
Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.
Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.
Sum the energies for step 1 and step 2.
This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.
Example Question #4 : General Thermodynamics
What was the final temperature of water if a 



Specific heat is the amount of heat needed to raise 1 gram of a substance by 1oC. Calorimeters used for these types of experiments because they are designed to be well-insulated, so no heat is gained from or lost to the surroundings.
Example Question #3 : Heat And Temperature
An 





Specific heat is the amount of heat needed to raise 1 gram of a substance by 1oC. Calorimeters used for these types of experiments because they are designed to be well-insulated, so no heat is gained from or lost to the surroundings.
Heat is transferred from the metal to the water.
Certified Tutor
Certified Tutor
All GRE Subject Test: Chemistry Resources



















































































