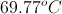All High School Physics Resources
Example Questions
Example Question #1 : Understanding Heat And Temperature
The temperature of an ideal gas is raised from 


For this problem, use Gay-Lussac's law to set up a direct proportion between pressure and temperature. Note that this law only applies when volume is constant.
Plug in our given values and solve for the initial pressure.
Example Question #11 : Heat





The formula for heat energy is:
We are given the initial and final temperatures, mass, and specific heat. Using these values, we can find the heat released. Note that the time is irrelevant to this calculation.
That means that the soup "lost" 
Example Question #1 : Understanding Heat And Temperature





The formula for heat energy is:
We are given the final temperature, mass, specific heat, and heat released. Using these values, we can find the initial temperature. Note that the time is irrelevant to this calculation. Since heat is released from the soup, the net change in the soup's energy is negative. Since the soup is cooling, we expect our answer to be greater than 
Example Question #2 : Understanding Heat And Temperature





The formula for heat energy is:
We are given the initial temperature, mass, specific heat, and heat released. Using these values, we can find the final temperature. Note that the time is irrelevant to this calculation. Since heat is released from the soup, the net change in the soup's energy is negative. Since the soup is cooling, we expect our answer to be less than 
Example Question #601 : High School Physics
An ice cube at 

Energy of the system remains the same
We would need to know the mass of the ice cube to draw a conclusion
Energy increases as the sample moves from a solid to a liquid
Energy is being used to convert the ice to water
The form of water has no bearing on the system energy
Energy is being used to convert the ice to water
When an object changes phase, it requires energy called "latent heat." In this case, even though the temperature is remaining constant, the energy inside of the ice cube is decreasing as it expends energy to melt.
Example Question #12 : Heat
A 



The equation for two items reaching a thermal equilibrium is given by describing a heat transfer. The heat removed from one object is equal to the heat added to the other.
We are given the specific heat values of each substance, as well as their masses. We also know the initial temperature of each substance. Use these terms in the equation to solve for the final temperature. Remember that the final temperature will be the same for each substance, since they will be in thermodynamic equilibrium.
Example Question #7 : Understanding Heat And Temperature
A sample of 



For this question, we must recognize that the system going to end up in equilibrium. That means that:
We are given the initial temperatures, masses, and specific heats of both the water and the ceramic. This will allow us to solve for the final temperature of the system; this value will be equal for both components. Notice that the specific heat given to us in the problem for the ceramic is in terms of kilograms, not grams. Convert to grams.
Example Question #22 : Thermodynamics
A 



We need to know the specific heat of the liquid in order to solve.
We need to know the freezing/boiling points of the liquid in order to solve.
The equation for change in temperature is
Plug in our given values.
Notice that the specific heats will cancel out.
Combine like terms.
Example Question #2 : Understanding Heat And Temperature
A 



The relationship between mass and temperature, when two masses are mixed together, is:
Using the given values for the mass and specific heat of each compound, we can solve for the final temperature.
We need to work to isolate the final temperature.
Distribute into the parenthesis using multiplication.
Combine like terms.
Certified Tutor
Certified Tutor
All High School Physics Resources