All AP Chemistry Resources
Example Questions
Example Question #1 : Composition Of Mixtures
Which of the following mixtures is not a homogeneous mixture:
Coffee
Tea
Oil and vinegar
Wine
A table salt solution
Oil and vinegar
A homogeneous mixture is a uniform mixture of two or more components that appears uniform throughout. The oil and vinegar mixture would not appear uniform, and thus is a heterogeneous mixture.
Example Question #2 : Composition Of Mixtures
Which of the following is a heterogeneous mixture.
Orange juice with no pulp
Gasoline
All of the above
Oatmeal chocolate chip cookie
Motor oil
Oatmeal chocolate chip cookie
A heterogeneous mixture is a mixture of two or more components that do not appears uniform throughout. The oatmeal chocolate chip cookie mixture would not appear uniform, and thus is a heterogeneous mixture.
Example Question #3 : Composition Of Mixtures
Which of the following are mixtures
Reeses Peanut butter cup
All of the above
b and c
Distilled water
Top soil
b and c
A mixture contains two or more different substances. Distilled water only contains liquid water, and is not a mixture.
Example Question #4 : Composition Of Mixtures
What differentiates a homogeneous mixture from a pure substance?
A mixture has a defined composition.
A pure substance is always a compound while mixtures can be elements or compounds.
The composition of a pure substance is fixed.
A pure substance can be separated into elements by physical means.
A homogeneous mixture is uniform throughout.
The composition of a pure substance is fixed.
A heterogeneous mixture is a mixture of two or more components that do not appears uniform throughout. A pure substance contains only one component that has a fixed composition.
Example Question #5 : Composition Of Mixtures
A slice of watermelon would best be described as?
None of the above
A heterogeneous mixture of elements and compounds
A homogeneous mixture of compounds
A heterogeneous mixture of compounds
A pure compound
A heterogeneous mixture of elements and compounds
A heterogeneous mixture is a mixture of two or more components that do not appears uniform throughout, but contains components that are elemental (single atom type) and compounds (contain two or more different elements).
Example Question #1 : Photoelectron Spectroscopy
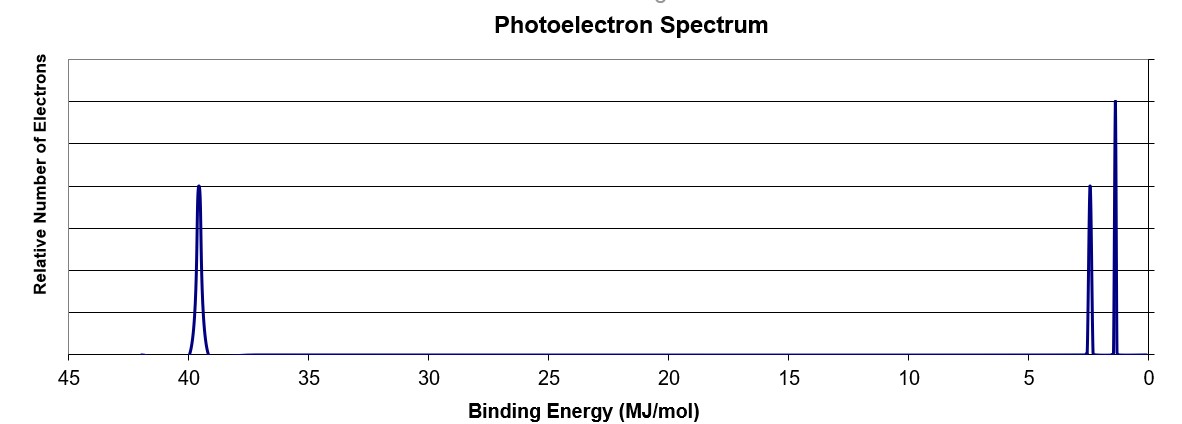
Using the PES spectra above, what element is illustrated?
N
Li
Na
C
Ne
N
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
Example Question #1 : Photoelectron Spectroscopy

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?
Mg is larger in size making the electrons in the 3s orbital farther from the nucleus and thus easier to remove
Na experiences a greater shielding effect from the 2s and 1s electrons making the 3s electrons easier to remove.
Na experiences a larger effective nuclear charge on the 3s electrons and are thus harder to remove.
None of the above
Mg experiences a larger effective nuclear charge and has more electrons in the 3s orbital.
Mg experiences a larger effective nuclear charge and has more electrons in the 3s orbital.
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Example Question #1 : Photoelectron Spectroscopy

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?
Nitrogen has 3 p-electrons and are thus unpaired leading to lower electron-electron repulsions.
Oxygen has more electrons and thus a greater amount of electron-electron repulsion present in the atom.
The effective nuclear charge on oxygen is greater than that of nitrogen.
There is a greater degree of electron shielding occurring in the oxygen 2p orbital.
Nitrogen is larger than oxygen thus making the 1s electrons easier to remove.
The effective nuclear charge on oxygen is greater than that of nitrogen.
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Example Question #1 : Photoelectron Spectroscopy
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1s peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
Example Question #10 : Atomic Structure And Properties

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?
None of the above
Step 1. Convert the Binding Energy from MJ/mol to J


Step 2:
Certified Tutor
All AP Chemistry Resources

![[He] 2s^22p^3](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172246/gif.latex)
![[Ne] 3s^2 3p^3](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172248/gif.latex)
![[Ne] 3s^23p^4](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172247/gif.latex)
![[He] 2s^2 2p^6](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172245/gif.latex)











