All Biochemistry Resources
Example Questions
Example Question #11 : Protein Structure And Functions
Enzymes can be regulated in a multitude of ways. One such way is by covalent modification, in which functional groups are attached to or removed from the enzyme. One such functional group that can be added to an enzyme is a phosphate group. Depending on the enzyme, addition of a phosphate group may increase or decrease that enzyme's activity. Which of the following is the general name of an enzyme that functions to add phosphate groups to its substrate?
Ligase
Isomerase
Kinase
Oxidoreductase
Phosphatase
Kinase
The correct answer is a kinase. Kinases are enzymes that couple the hydrolysis of ATP to the addition of a phosphate group to its substrate.
Phosphatase enzymes basically function oppositely to how kinases work. Phosphatases use water to hydrolyze phosphate groups off of their substrate.
Isomerase enzymes function to interconvert the structure of molecules from one isomer to another. This means that the substrate will remain with the same molecular formula, but it will have a difference in the connectivity of its bonds.
Ligases are enzymes that work by joining two molecules together.
Oxidoreductases are enzymes that act by catalyzing oxidation and reduction reactions, which involve the transfer of electrons from one molecule to another.
Example Question #1 : Transferases And Kinases
In the last step of glycolysis, what is the name of the enzyme that converts phosphoenolpyruvate into pyruvate?
Pyruvate kinase
Hexokinase
Aldolase
Phosphoglycerate kinase
Phosphofructokinase-1
Pyruvate kinase
The last step of glycolysis, which involves the conversion of phosphoenolpyruvate into pyruvate, is catalyzed by the enzyme pyruvate kinase, yielding one pyruvate molecule and 1 ATP. The other kinases are involved in different steps of glycolysis.
Example Question #2 : Transferases And Kinases
Which of the following enzymes catalyzes a reaction that is the functional opposite of the reaction catalyzed by kinases?
Endonuclease
Flippase
Phosphatase
Lipase
None of these
Phosphatase
Kinases catalyze the attachment of phosphate groups to their substrates. Phosphatases specifically remove phosphate groups from their substrates, which is the opposite of the function of kinases. The other enzymes listed do not have functions that involve removal of phosphate groups.
Example Question #12 : Protein Structure And Functions
Which of the following is false regarding protein kinase A (PKA)?
When activated, PKA undergoes conformational changes and phosphorylates its targets
cAMP binds to a regulatory domain and inhibits kinase activity
PKA is comprised of 2 catalytic domains that are sequestered by 2 regulatory domains
PKA utilizes ATP
cAMP binds to a regulatory domain and inhibits kinase activity
From it's name, we can assume that this kinase will add phosphate groups to its targets. The source of these phosphate groups is ATP. PKA has 2 catalytic, and 2 regulatory subunits. When PKA is activated, there is a conformational change that causes the regulatory subunits to fall off, and frees the catalytic (kinase) portions. When PKA is inhibited, the regulatory subunits bind to the catalytic subunits and prevent phosphorylation. PKA has many activators, but cAMP is one of the most robust and well studied activator. Therefore, cAMP binds to the regulatory subunits, but does not inhibit protein function.
Example Question #1 : Protein Functions
Kinases catalyze the phosphorylation of other proteins/substrates, which may trigger their activation. Phosphorylation involves the addition of a phosphate group to the target molecule. Amino acids with a(n) __________ R-group are typically the substrates for phosphorylation.
Aromatic
Biogenic
Non-polar
Polar
Chiral
Polar
Polar R-groups, namely free hydroxyl groups on serine, threonine, and tyrosine, are the usual targets for phosphorylation because they are nucleophilic and can react with the phosphate group. Other R-groups are not as reactive and therefore are not ideal sites for phosphorylation.
Example Question #3 : Transferases And Kinases
Which of the following is false about cyclin-dependent kinases (Cdks)?
Cdk binds to a single cyclin throughout the entire cell cycle.
Vertebrate cells have four types of Cdks.
Cdk activity rises as mitosis starts.
Cylcin proteins control the activity of Cdks.
In yeast cells, one Cdk protein regulates the cell cycle.
Cdk binds to a single cyclin throughout the entire cell cycle.
One of the features of the beginning of mitosis, and various steps in the cell cycle, is the increase of cyclin activity. This cyclin controls the action of Cdk, causing changes in the phosphorylation of proteins, influencing cell cycle events. Yeast cells use one Cdk, which changes cyclin throughout the cycle, while vetrebrate cells use four -- 

Example Question #31 : Biochemistry
Lysosomal enzymes are predominantly __________.
oxidases
decarboxylases
isomerases
hydrolases
kinases
hydrolases
The lysosome is the "stomach" of the cell. It contains many hydrolytic enzymes to digest and recycle the monomers used to form old polymers. Remember the opposite of dehydration/condensation synthesis is hydrolysis. Hydrolysis reactions use water to break bonds in polymers, yielding monomers that can be recycled and reused in anabolic pathways.
Example Question #2 : Hydrolases
Which of the following best describes how a lysozyme works?
It hydrolyzes bonds in lipids, causing a split in a fatty acid chain.
It cuts a polysaccharide relatively slowly, facilitating a random, spontaneous collision between water and the sugar, with little intervention.
It is responsible for the cleaving of amino acid chains via the ping-pong mechanism.
It cuts the bond in a polysaccharide, by holding six sugars in a row in its active site, and adding a water molecule, causing hydrolysis.
It cleaves the phosphodiester bond in nucleic acids, via hydrolysis.
It cuts the bond in a polysaccharide, by holding six sugars in a row in its active site, and adding a water molecule, causing hydrolysis.
Lysozymes speed up by many times the hydrolysis of polysaccharides, by adding the water molecule to sugars linked in its enzyme-substrate complex. If left alone without the lysozyme, this hydrolysis would occur relatively infrequently, because it requires a large activation energy which would be supplied only by rare random collisions. The amino acid cleavage enzyme which uses the ping-pong mechanism is chymotrypsin. The enzyme which breaks nucleic acid phosophodiester bonds is phosphodiesterase. Fats are hydrolyzed by lipases, not lysozymes.
Example Question #31 : Biochemistry
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
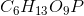
Based on this action, to which enzyme class does phosphoglucomutase belong?
Ligase
Oxidoreductase
Isomerase
Lyase
Isomerase
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Example Question #32 : Biochemistry
Which of the following correctly mentions the function of a common eukaryotic ligase?
Join lagging strands (Okazaki fragments) of DNA during replication
Transfer amino groups from an amino acid to an alpha-keto acid
Convert adenosine to adenine and ribose in the presence of water
Catalyze the conversion of glucose-6-phosphate into fructose-6-phosphate
Convert pyruvate into acetyl-coenzyme A
Join lagging strands (Okazaki fragments) of DNA during replication
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Certified Tutor
Certified Tutor
All Biochemistry Resources




