All College Chemistry Resources
Example Questions
Example Question #1 : Common Ion Effect
Calculate the molar solubility of barium fluoride when it is dissolved in a solution containing 


Start by writing out the equation for the dissolution of barium fluoride.
Next, write out the chart to keep track of concentrations of each ion.
|
|
[ |
[ |
|
Initial |
0.00 |
0.200 |
|
Change |
+x |
+2x |
|
Equilibrium |
x |
0.200+2x |
In the chart, 


Now, write the equilibrium expression using the chemical equation:
Plug in the given 

At this point, use a graphing calculator to solve for 
The molar solubility of barium fluoride in sodium fluoride is 
Example Question #133 : College Chemistry
Suppose that a chemist wants to lower the concentration of calcium in an aqueous solution by precipitating it out, according to the following reaction expression.
Which of the following chemicals could the chemist add to the solution in order to precipitate the calcium?
Any of these
In this question, we're shown an equilibrium expression for a precipitation reaction in solution. We're asked to identify a compound that will help calcium precipitate out of solution.
To identify a compound that will precipitate calcium out of solution, we need to consider the equilibrium expression shown in the question stem. Applying Le Chatlier's principle, we'll need to consider the direction each of the answer choices will push the reaction in.
Adding 

Adding 
Adding 
Adding 


Certified Tutor
All College Chemistry Resources




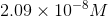

 ]
] ]
]![\text{K}_{sp}=[\text{Ba}^{2+}][\text{F}^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/902868/gif.latex)










