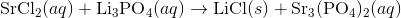All College Chemistry Resources
Example Questions
Example Question #13 : Solutions, States Of Matter, And Thermochemistry
If a liquid has a low resistance to flow, it has a low __________.
Viscosity
Melting point
Boiling point
Volume
Vapor pressure
Viscosity
By definition, viscosity is the measure of a liquid's resistance to flow. If a liquid has a low viscosity, it has a low resistance to flow. It may not necessarily always have a low boiling point, melting point, and/or vapor pressure due to this characteristic. Viscosity is just a way to measure and describe one physical characteristic (resistance to flow) of a liquid. The volume of a liquid does not say anything about its physical properties.
Example Question #1 : Liquids And Solutions
Write an equation for the precipitation reaction that occurs (if it occurs) when a solution of strontium chloride is mixed with a solution of lithium phosphate.
Start by writing out the the chemical formulas for the reactants:
In order to figure out the possible products, combine the cation of one molecule with the anion of the other molecule.
For this reaction, the possible products are 

Next, use solubility rules to figure out if any precipitate is formed. Since compounds with 




Thus, we can write the final chemical equation:
Example Question #3 : Liquids And Solutions
Which of the following is insoluble in water?
Recall the solubility rules:


Example Question #4 : Liquids And Solutions
Which of the following substances is insoluble in aqueous solution?
Solubility rules can help us find the answer to this question.
First, solubility rules state that all compounds of Group 1A elements on the periodic table are soluble in aqueous solution. This means that all of the alkali metals, including potassium, form compounds which are soluble in aqueous solution; thus, 
Solubility rules also tell us that all ammonium salts (salts of 

Next, solubility rules tell us that all bromide salts are soluble, except for those of 




Example Question #5 : Liquids And Solutions
Which of the following is not readily soluble in water?
Remember the solubility rules for ionic solids in water:
1) Salts of group 1 (with few exceptions) and NH4+ are soluble
2) Nitrates, acetates, and perchlorates are soluble
3) Salts of silver, lead, and mercury (I) are insoluble
4) Chlorides, iodides, and bromides are soluble
5) Carbonates, phosphates, sulfides, oxides, and hydroxides are insoluble. Exceptions: sulfides of group 2 cations and hydroxides of calcium, strontium, and barium are slightly soluble
6) Sulfates are soluble except for those of calcium, strontium, and barium
Following these rules, we see that MgOH is insoluble in water
Example Question #1 : Liquids And Solutions
A solution is prepared by dissolving 


Recall how to find the molality of a solution:
First, start by finding the moles of glucose that we have. The molar mass of glucose is 
Next, convert the grams of water into kilograms.
Now, plug in the moles of glucose and kilograms of water into the equation for molality.
Example Question #2 : Molarity, Molality, Normality
A solution of hydrogen peroxide is 

Start by assuming that we have 

Use the given density to find the mass of the solution.
Next, find the mass of the hydrogen peroxide present in the solution.
Convert the mass of hydrogen peroxide into moles of hydrogen peroxide.
Recall how to find the molarity of a solution:
Since we have 

Example Question #3 : Molarity, Molality, Normality
What is the molarity of a solution in which 




The first step is to calculate how many moles of 
We calculate molarity with the following equation:
Example Question #4 : Molarity, Molality, Normality
How many milliliters of a 






We can use the formula
Therefore,
Example Question #5 : Molarity, Molality, Normality
What are the concentrations of aluminum and sulfate in a 3.0 M solution of aluminum sulfate?
The relative concentrations of aluminum sulfate 
All College Chemistry Resources