All MCAT Physical Resources
Example Questions
Example Question #1 : Spectroscopy
An IR spectroscopy reading of a compound shows a large absorption at 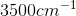
Possible Answers:
Amine
Amide
Aldehyde
Alcohol
Correct answer:
Alcohol
Explanation:
Compounds with an alcohol group show absorptions in an IR sprectrum from 








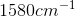



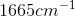
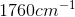
Karen
Certified Tutor
Certified Tutor
Case Western Reserve University, Bachelor of Science, Chemical Engineering. Case Western Reserve University, Master of Arts, ...
All MCAT Physical Resources
Popular Subjects
Spanish Tutors in Boston, SAT Tutors in Washington DC, GMAT Tutors in Philadelphia, Physics Tutors in San Diego, Biology Tutors in Atlanta, SAT Tutors in San Diego, Algebra Tutors in Philadelphia, LSAT Tutors in Atlanta, MCAT Tutors in Philadelphia, MCAT Tutors in San Diego
Popular Courses & Classes
ISEE Courses & Classes in San Diego, SAT Courses & Classes in Los Angeles, ISEE Courses & Classes in San Francisco-Bay Area, Spanish Courses & Classes in Chicago, ACT Courses & Classes in Chicago, ISEE Courses & Classes in Atlanta, GRE Courses & Classes in New York City, SAT Courses & Classes in Philadelphia, SSAT Courses & Classes in Denver, MCAT Courses & Classes in Miami
Popular Test Prep
ACT Test Prep in Washington DC, SAT Test Prep in Phoenix, MCAT Test Prep in Atlanta, MCAT Test Prep in New York City, MCAT Test Prep in Washington DC, GRE Test Prep in New York City, MCAT Test Prep in Dallas Fort Worth, GRE Test Prep in Houston, ACT Test Prep in Chicago, ISEE Test Prep in San Diego




