All Organic Chemistry Resources
Example Questions
Example Question #3 : Mechanisms And Intermediates
For which of the following acid-base reactions will the equilibrium lie on the left side?

The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.
Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
Example Question #71 : Organic Concepts
A carboxylic acid has a pKa of 5. At a pH of 8, what is the ratio of salt to acid?
Use the Henderson Hasselbalch equation:
Example Question #2 : Help With Acid Base Reactions

List the given compounds in order of decreasing basicity.
II, III, I, IV
II, I, III, IV
II, III, IV, I
III, II, IV, I
IV, I, III, II
II, I, III, IV
An easy way to consider relative base strengths is to consider the strength of the compounds' conjugate acids.The stronger the conjugate acid, the weaker the base. Water (compound IV) is the least basic of the compounds because its conjugate acid, 






Example Question #1 : Help With Acid Base Reactions

Rank these weak acids by decreasing 
IV, I, II, III
III, IV, II, I
III, IV, I, II
II, III, I, IV
IV, III, I, II
III, IV, II, I
The governing principle regarding the prediction of 




Example Question #2 : Help With Acid Base Reactions
A.
B.
Which of the above molecules is expected to have a more acidic alpha-carbon, and why?
Molecule A, because its conjugate base has less stability.
Molecule B, because its conjugate base has less stability.
Molecule B, because its conjugate base has more stability.
Molecule A, because its conjugate base has more stability.
Molecule B, because its conjugate base has more stability.
Molecule B will have a more acidic alpha-carbon because once the alpha proton becomes dissociated, the conjugate base will have relatively more stability than the conjugate base of molecule A.
When the alpha-carbon on molecule A loses it's proton, the conjugate base is not as stable. The reason for this is because the oxygen that is involved in the ester bond can contribute its electrons towards a resonance structure. Therefore, after the alpha-proton is lost, the alpha-carbon will have a negative charge that will be destabilized by the delocalized negative charge of the resonance structures.
Example Question #76 : Organic Concepts
Which of the following is the strongest acid?
From the start, we know we can eliminate answer choice 


Example Question #7 : Help With Acid Base Reactions
Which of the following sets of bases are listed from most basic to least basic?
The correct ranking from most basic to least basic is:
The best bases are negatively charged, and the worst bases are positively charged (acidic). The stronger the base, the weaker (more stable) it's conjugate acid. An alkane is a very stable conjugate acid, which tells us that 
We know based on charge alone, that 



We know that 




Example Question #53 : Reactions Types
Which of the following sets of acids are correctly listed from most to least acidic?
Remember that the strongest acids have the weakest conjugate bases. 




Example Question #1 : Help With Acid Base Reactions

Rank the given molecules in order of increasing pKa.
III, I, IV, V, II
I, II, V, IV, III
III, II, V, I, IV
II, V, IV, I, III
IV, II, V, I, III
II, V, IV, I, III
Recall that the stronger an acid, the lower the pKa.
II (two fluorine atoms really close to 
V (one fluorine really close to 
I (one fluorine a little further away from 
IV (no inductive effect)
III (alcohols are much less acidic than carboxylic acids, and it has the highest pKa of all)
Example Question #2 : Help With Acid Base Reactions
Which side (left or right) of the following reaction is favored and why?

Right side, because the 


Left side, because the 


Left side, because the 


Right side, because the 


Right side, because the 


The side of the reaction that is favored will have the acid with the higher 

Certified Tutor
All Organic Chemistry Resources



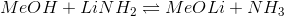






![pH = pKa + log \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439185/gif.latex)
![8 = 5 + log \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439186/gif.latex)
![10^{3} = \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439187/gif.latex)



















