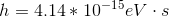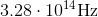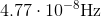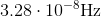All Physical Chemistry Resources
Example Questions
Example Question #1 : Quantum Chemistry
Which of the following quantities is zero for photons?
Mass
Neither mass nor speed
Both mass and speed
Speed
Mass
Photons are fundamental particles that make up electromagnetic waves (light). The key aspect of photon is that it has very high speed 
Example Question #1 : Quantum Chemistry
Which of the following is/are the same for photons in gamma rays and in visible light?
I. frequency
II. wavelength
III. speed
III only
I and II
I only
None of these
III only
Electromagnetic spectrum is a spectrum of different types of light. Each type of electromagnetic wave differs from one another based on the frequency and wavelength; however, the speed of every electromagnetic wave in air is 
Here, 



Example Question #1 : Quantum Chemistry
Photons in ultraviolet rays have a wavelength of 10nm. What is the energy of photons in radio waves?
To solve this question, we need to use the equation relating energy of a wave to its wavelength.
Here, 




If we look at the electromagnetic spectrum we will notice that the wavelength of radio waves is a lot higher than ultraviolet rays. Since energy is inversely proportional to wavelength we can conclude that the energy of radio waves is lower than ultraviolet's. The only answer choice with an energy lower than ultraviolet rays energy is 1.24neV.
Example Question #1 : Emission And Absorption Spectra
After undergoing emission a molecule is __________ stable and after undergoing absorption a molecule is __________ stable.
less . . . more
more . . . less
more . . . more
less . . . less
more . . . less
Emission is the process of releasing (or emitting) photons from an atom whereas absorption is the process of absorbing photons. After emission, the atom releases energy (in the form of photons) and goes to a lower energy state. In absorption, however, the atom absorbs energy and goes to a higher energy state. Recall that lower energy always means more stable; therefore, after emission, an atom or molecule goes to a more stable state whereas after absorption it goes to a less stable state.
Example Question #1 : Electromagnetic Spectrum And Radiation
The wavelength of a particular color of red-orange light is 
What is the frequency of this color?




Example Question #1 : Quantum Chemistry
Microwave radiation falls in the wavelength region of 
What is the frequency of microwave radiation that has a wavelength of 





Example Question #2 : Quantum Chemistry
A researcher analyzes two types of electromagnetic waves. He observes that wave A has a higher amplitude than wave B. What can you conclude about these two waves?
Wave A has the higher wavelength
Wave A has the higher speed
Wave B has the higher wavelength
The relative wavelengths and speed cannot be determined without knowledge of the medium through which the wave is traveling.
The relative wavelengths and speed cannot be determined without knowledge of the medium through which the wave is traveling.
Amplitude of a wave is defined as the maximum peak of oscillation or vibration. Recall that frequency, wavelength, and speed are not dependent on the amplitude and are calculated using the wave equation:
Here, 


Example Question #2 : Nuclear, Quantum, And Molecular Chemistry
If a star approaches earth the electromagnetic waves from the star are perceived to have shorter wavelength whereas if a star moves away from the earth the waves have longer wavelength.
When observing through a telescope, a scientist notices that star A appears red whereas star B appears blue. Which of the following is true regarding these two stars?
More than one of these are true
Star B is approaching earth
Star A is approaching earth
The number of photons from both stars is approximately the same
Star B is approaching earth
The phenomenon described in this question is called redshift and blueshift. All stars emit electromagnetic waves. The perceived wavelength of these waves depends on the relative motion of the object. If an object is approaching the observer (in this case, the earth) then the apparent wavelength of emitted waves decreases whereas if the object moves away then the apparent wavelength increases. This is called the Doppler effect.
To answer this question, we need to know the relative wavelengths of blue and red light. Blue light has wavelength between 

Recall that lower wavelength means higher frequency; therefore, blue has the higher frequency.
Example Question #1 : Nuclear, Quantum, And Molecular Chemistry
Microwave rays have a __________ wavelength and a __________ energy than ultraviolet rays.
lower . . . lower
higher . . . higher
lower . . . higher
higher . . . lower
higher . . . lower
To solve this question, we need to first recall the electromagnetic spectrum. Microwaves have very large wavelengths. They are found between visible light and radio waves. Ultraviolet, on the other hand, is found below visible light; therefore, ultraviolet rays have smaller wavelength than microwaves.
To solve for energy we need to use the following equation.
Here, 



Gamma rays have the lowest wavelength and highest energy whereas radio waves typically have the highest wavelength and lowest energy.
Example Question #2 : Electromagnetic Spectrum And Radiation
Two light sources are placed equidistant from a detector. The light from light source A is detected quicker than light from light source B. What can be concluded from these results?
Source A has a higher wavelength
Source A could produce red light whereas source B could produce blue light
The number of photons per unit time is higher from source A
The results are invalid
The results are invalid
The question states that there are two light sources and that both are placed equidistant from a detector. This means that any differences in detection speed is not related to the distance. The question also states that the detector picks up light from source A quicker that light from source B. This means that the speed of the light from source A is faster. Recall that light (or electromagnetic rays) can have varying wavelengths and frequencies; however, the speed of all electromagnetic rays is the same. It doesn’t matter if it is gamma rays or visible light, the speed of light in air is 
We cannot determine the color of the light for the light sources because we don't have any information regarding their respective wavelength or frequency. The number of photons per unit time refers to the amplitude of the wave, which also cannot be determined from the given information.
Certified Tutor
All Physical Chemistry Resources