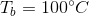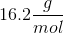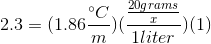All Physical Chemistry Resources
Example Questions
Example Question #1 : Boiling Point
What is the boiling point of a 100mL solution of water after the addition of 29g of sodium chloride?
We can use the boiling point elevation equation in order to find the new boiling point for the solution.
The change in temperature will be equal to the boiling point elevation constant multiplied by the molality of the solution multiplied by the van't Hoff factor. The van't Hoff factor is a variable that incorporates how many ions the added solute will dissolve into when in solution. Sodium chloride will dissolve into two ions, so the van't Hoff factor is 2.
This means that the boiling point of the water has been elevated by 5.2 degrees, meaning that the boiling point of the solution is 105.2 degrees celsius.
Example Question #2 : Colligative Properties
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.

We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
Example Question #61 : Physical Chemistry
Which of the following is not an intensive property?
Melting point
Pressure
Volume
Temperature
Density
Volume
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Certified Tutor
Certified Tutor
All Physical Chemistry Resources