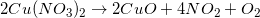All AP Chemistry Resources
Example Questions
Example Question #2 : Types Of Reactions
The reaction above could be classified as __________.
single displacement
synthesis
decomposition and oxidation-reduction
oxidation-reduction
decomposition
decomposition
When one molecule is converted into two or more smaller molecules, the reaction is considered a decomposition reaction. In this example, none of the elements under go a change in oxidation number, so this is not an oxidation-reduction reaction. (Calcium is always 


Example Question #1 : Introduction For Reactions
Which of the following represents a combustion reaction?
Combustion reactions always produce 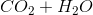
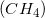

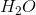
Example Question #2 : Introduction For Reactions
What is the chemical formula of the salt formed when a chemist mixes solvated Potassium and Arsenic ions in solution?
Potassium is a Group I element, so to get to a filled valence shell, it will lost one electron, yielding 
Arsenic is a Group 5 element, so it needs to gain three electrons to obtain a filled valence shell, yielding 
In order to balance out the charges, the resultant salt will be 
Example Question #1 : Introduction For Reactions
What is the net ionic equation for the ion exchange reaction between ferrous sulfate and calcium iodide? Assume all compounds are soluble.
None of the available answers
First, we must know what ferrous sulfate is. Ferrous refers to 


Calcium is a divatent cation and iodide is a monovalent anion, so their salt is 
Example Question #1 : Balancing Chemical Equations
Select the net ionic equation from this molecular reaction:
None of the other choices
The net ionic equation is derived by removing all spectator ions from the total ionic equation (in which all ions are listed). To put it another way, the net ionic equation involves only the ions that participate in a reaction which, in this case, is the precipitation of barium sulfate.
Begin by writing all aqueous compounds in their dissociated (ionic) forms.
Cancel out any ions that appear in equal quantities on both sides of the equation. In this case, we can cancel the nitrate and potassium ions.
This is our net ionic equation.
Example Question #2 : Balancing Chemical Equations
What is the balanced chemical equation for the combustion of butane 
Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:
We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.
Similarly, we balance carbons by putting a 4 on the carbon dioxide.
To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for 
Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:
Example Question #4 : Introduction For Reactions
Determine whether or not solid aluminum reacts with aqueous zinc chloride. If it does, determine the balanced equation for the reaction.
No reaction occurs
When we check the activity series, it is fairly easy to see that aluminum metal is more reactive than zinc metal. So, in this case, the two metals undergo a redox reaction, where the aqueous 



Because 

We note that there are 2 chlorine atoms on the left and 3 chlorine atoms on the right. To balance, we use a 3 coefficient on the left and a 2 coefficient on the right. This gives a total of 6 chlorine atoms on eahc side.
However, now we have also increased the amounts of zinc and aluminum. We copy the necessary coefficients to balance those—2 for aluminum on the left, 3 for zinc on the right—and we are done:
Example Question #5 : Introduction For Reactions
What is the net ionic form of this equation?
To find the net ionic form of this chemical equation, first balance the equation, then dissociate all soluble components. In other words, separate the aqueous terms into two separate molecules/atoms. For example, 



Write out the dissociated forms of each species, if applicable.
Cross out species that appear on both sides of the equation. The net ionic equation is:
Example Question #6 : Introduction For Reactions
What is the coefficient for 
The best way to solve this problem is to try all the possible answers. When we try 8, 






Example Question #1001 : Ap Chemistry
Given that lithium is higher on the activity series than hydrogen, what should be seen when solid lithium and hydrochloric acid are mixed?
None of these
Bubbles
No change
Increase in test tube's temperature
Change in color
Bubbles
Given that lithium is more reactive than hydrogen, expect a single replacement reaction to take place, so lithium will replace hydrogen.
All ionic compounds are solids at room temperature, which is why lithium chloride has the solid phase label. Remember that hydrogen is a diatomic element which is a gas a room temperature. When observing reactions in which gasses are produced from a solution, bubbles are seen. There is no indication whether this reaction is endothermic or exothermic, nor is there any information that suggests the reaction should change color.
All AP Chemistry Resources