All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Hydrocarbon Products
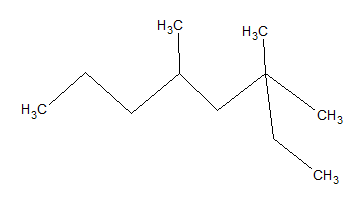
What is the IUPAC name of the given molecule?
3,3,5-trimethylnonane
3,3,5-trimethyloctane
None of these
2,2,4-trimethyloctane
4,6-dimethyl-6-ethylpentane
3,3,5-trimethyloctane
The longest carbon chain that can be formed is eight carbons. The base molecule is octane.
Using IUPAC rules, substituents should have the lowest possible numbers; thus, we start counting carbons from the right side rather than the left. If you count from the correct side, there are two methyl groups on carbon 3 and one on carbon 5. Thus, the name of the moleculue is 3,3,5-trimethyloctane.
Example Question #2 : Hydrocarbons

How could you brominate the compound?
None of these
Bromine gas
Bromine and UV light
Bromine and peroxides
Hydrobromic acid
Bromine and UV light
The given molecule is an alkane. The only way to brominate an alkane is with bromine gas and UV light. The energy from the light serves to creat two radical bromines. These radicals are capable of bonding with alkanes. If the given compound were an alkene, either hydrobromic acid or bromine and peroxides would work.
Example Question #3 : Hydrocarbons
Which of the following can reduce an alkene to an alkane?
Lithium aluminum hydride (LiAlH4)
H2/Pd and H2/Raney nickel
H2/Raney nickel
H2/Pd
Lithium aluminum hydride (LiAlH4) and H2/Pd
H2/Pd and H2/Raney nickel
Neither lithium aluminum hydride, nor sodium borohydride will reduce C–C double bonds.
H2/Raney nickel and H2/Pd can each (individually) reduce an alkene to an alkane. Since both H2/Raney nickel and H2/Pd can reduce the alkene, the answer is both of those reagents. This is a catalytic hydrogenation reaction, and H2/Raney nickel not only reduces C–C double bonds, but also carbonyl compounds.
Example Question #1 : Functional Group Reactions
Identify the major organic product expected from the acid-catalyzed dehydration of 2-methyl-2-pentanol.
2-methyl-2-pentene
cis-3-methyl-2-pentene
None of the other answers
3-methyl-1-pentene
2-methyl-1-pentene
2-methyl-2-pentene
The initial compound is a five-carbon alkane chain with methyl and hydroxy groups on the second carbon. Dehydration involves the hydrogenation of the hydroxy group. That group then leaves, and a double bond is formed. Zaitsev's rule states that double bonds are more stable on more highly substituted carbons. The double bond forms across carbons two and three.
Certified Tutor
All GRE Subject Test: Chemistry Resources




