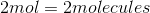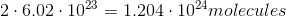All High School Chemistry Resources
Example Questions
Example Question #1 : High School Chemistry
What unit will be used to denote temperature in thermochemistry?
Celsius
Kelvin
Fahrenheit
Celsius or Kelvin
Joules
Kelvin
Temperature is the measure of the average kinetic energy of a system. Kelvin is the SI unit for temperature, and must be used because it is the only scale that does not have any negative numbers possible. At zero Kelvin (absolute zero) and there is absolutely no movement in the system, down to the atomic level.
While temperature is a means of measuring energy in a system, actual heat energy has the unit Joules.
Example Question #2 : Measurements
Which of the following measurements contains 4 significant figures?
1. Any digit with a value 1-9 is always a significant figure
2. Any 0 between two digits 1-9 is always significant
3. If a number is greater than 1 and does not have a decimal point a 0 at the end is not significant because it is a tailing zero
4. If a number is less than 1 any 0 before a digit 1-9 is not significant because it is a leading zero
5. If there is a decimal point in any number, any 0 that follows a non-zero digit is always significant.
Thus, 
Example Question #3 : High School Chemistry
Which has the larger unit listed first?
Microsecond and nanosecond
Picometer and millimeter
Centigram and decigram
Decaliter and kiloliter
Kilojoule and megajoule
Microsecond and nanosecond
The following prefixes are ranked in order from largest to smallest with the power of 10 that the unit represents: peta (P) 














Example Question #2 : Measurements
The measurement of 
Units in the metric system can be converted using the correct factor of 10.
Example Question #1 : Using Si Units
A certain metal is weighed on a balance in a weighing boat. Before the metal is added, the mass of the weigh boat is recorded at 



What is the density of the metal?
Density is equal to the mass of the object divided by the volume.
The mass of the metal is figured out by taking the mass of the metal and the weighboat 
The volume of the water is calculated by the displacement of the water so take the final volume and subtract the initial volume.
The density of the metal is calculated by taking the mass divided by the volume.

Example Question #3 : High School Chemistry
An object is measured to be 
You must use equalities and dimensional analysis to solve for the correct answer:
Each equality allows you to write a conversion factor 2 ways:
Example: 

To cancel out units if there is a unit on top it must be cancelled out by the same unit on the bottom.
To solve you must cancel out units until you get to the proper unit
Example Question #2 : High School Chemistry
Consider the following four samples:




Which of the given samples contains the most atoms?
Chlorine
Potassium
They all have the same number of atoms
Lithium
Lithium
It is important to note that the mass of a sample does not tell you the amount of atoms in the sample. The number of atoms in a sample is dependent on the number moles in a sample, given by Avogadro's number. Here is the number of moles for each sample:
Remember that chlorine is a diatomic mass, so each molecules contains two atoms. This doubles the molar mass for the conversion.
The sample with the greatest number of moles will also contain the most atoms. In this case, the sample of lithium results in the largest number of moles and, thus, the greatest number of atoms.
Example Question #4 : High School Chemistry
Consider the reaction above. If you start with 
In the chemical equation, the ratio of potassium bromide to bromine is 2:1, so for every 2 moles of 
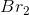


Example Question #5 : High School Chemistry
Convert the following amount from grams (g) to moles (m)
How many moles is 

Use the periodic table to calculate the molecular weight of sodium hydroxide.
Next, use dimensional analysis to find the number of moles.
Example Question #6 : High School Chemistry
How many moles of 

The molecular weight of carbon dioxide is 

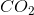

All High School Chemistry Resources