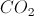All MCAT Biology Resources
Example Questions
Example Question #1 : Reaction Types
Which of the following statements is true concerning the Hofmann elimination reaction?
The least substituted alkene is the major product in the reaction
The Zaitsev product is favored in the elimination reaction
The ammonium is eliminated following an E1 mechanism
The quaternary ammonium salt is a poor leaving group
The least substituted alkene is the major product in the reaction
The Hofmann product is the most favored product in a Hofmann elimination reaction. The reaction follows an E2 mechanism, where a quaternary ammonium salt is able to be removed from a hydrocarbon, resulting in an alkene product. This reaction results in the least substituted alkene being the primary product.
Example Question #21 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following statements is false concerning the Wittig reaction?
The ketone or aldehyde is bound by the negatively charged phosphorous atom
The product compound that includes the phosphorous from the ylide will be attached to the reactant's carbonyl oxygen
A mixture of cis and trans isomers are created in the reaction
The ylide attaches to the substrate via nucleophilic addition
The ketone or aldehyde is bound by the negatively charged phosphorous atom
The Wittig reaction is used to make an alkene from a ketone or aldehyde. The ylide (RPPh3) attaches to the substrate via nucleophilic addition, with the negative carbanion attaching to the ketone or aldehyde. The phosphorus atom carries a positive charge, balancing the carbanion.
All other listed answer choices are true. The products of the reaction are an alkene (cis and trans) with the addition of the R group of the ylide and Ph3PO.
Example Question #1411 : Mcat Biological Sciences
All of the following are characteristics of a Wittig reaction except __________.
it proceeds through a phosphaoxetane intermediate
it involves the reaction of a phosphonium ylide with a carbonyl
it results in the exclusive formation of trans double bonds
it produces a trialkylphosphine oxide or triarylphosphine oxide as a by-product
it results in the formation of a carbon-carbon double bond
it results in the exclusive formation of trans double bonds
The Wittig reaction involves the reaction of a phosphonium ylide (generated by treating a phosphonium salt with a strong base) with a ketone or aldehyde.
The reaction proceeds through a phosphaoxetane (4-membered ring containing both phosphorus and oxygen) intermediate to generate a new compound containing a carbon-carbon double bond, plus a phosphine oxide byproduct. It does not form trans double bonds exclusively; sometimes, a mixture of cis and trans isomers are obtained, and sometimes the cis isomer is the predominant product.
Example Question #2 : Reaction Types
What is created when a ketone is reacted with a phosphorus ylide?
Alkane
Ester
Aldehyde
Alkene
Alkene
The Wittig reaction involves a ketone or aldehyde reacting with a phosphorus ylide, a molecule with a negatively charged carbanion. The ketone will undergo nucleophilic addition and form a betaine. This intermediate will then form an alkene with a triphenylphosphine oxide being released. The Wittig reaction will form a mixture of both cis and trans isomers if the carbanion has two different substituents.
Wittig general reaction:
Example Question #1 : Reactions With Ketones And Aldehydes
The reaction between one mole of acetone and one mole of 1-propanol in aqueous acid will result in the formation of what product?
A hemiketal compound
An acetal compound
A hemiacetal compound
A ketal compound
A hemiketal compound
A hemiketal compound is the result of nucleophilic attack by an alcohol (1-propanol) on the carbonyl of a ketone (acetone). The previously double-bonded oxygen now bears a negative charge, and deprotonates the now positively charged attacking alcohol. The hemiketal of acetone will have a hydroxy group and an -OCH3 group bound to the central carbon.
Example Question #23 : Organic Chemistry, Biochemistry, And Metabolism
An amide compound
No reaction occurs
An enamine
An amino compound
An imine
An imine
This reaction shows nucleophilic acyl attack on a carbonyl group by the ammonia molecule. This reaction leads to the loss of oxygen as water, and eventually the formation of an imine. Note the difference between each product type. An imine is a nitrogen with one nitrogen-carbon double bond and one substituent. An enamine is formed from a nitrogen with no double bonds. An amide is a nitrogen bound to a carbonyl carbon.
Example Question #3 : Reaction Types
An aldehyde undergoing one round of nucleophilic attack by an equivalent of alcohol results in a(n) __________ product, where two rounds of nucleophilic attack (two alcohol equivalents) results in a(n) __________ product.
hemiketal . . . ketal
acetal . . . ketal
acetal . . . hemiacetal
hemiacetal . . . acetal
hemiacetal . . . acetal
The correct answer is hemiacetal and acetal. The partial positive character of a carbonyl carbon makes it susceptible to nucleophilic attack. An alcohol's oxygen has free electrons, and therefore can serve as a nucleophile. As the bond between the attacking alcohol's oxygen and the carbonyl carbon forms, the pi bond of the carbonyl group is lost. Once the attacking alcohol's oxygen is deprotonated, the resulting product is an ether (from the attacking alcohol) and a new hydroxyl group (from the now protonated carbonyl oxygen). The resulting molecule is referred to as the hemiacetal product.
Subsequent addition of alcohol leads to the loss of the hydoxyl group as water, resulting in the second ether chain to the original carbon, which is referred to as the acetal product.
The same reaction takes place with ketones as well. With ketones, the first addition of alcohol results in the hemiketal product, and the second addition of alcohol results in the ketal product.
It is important to remember the following material for analyzing acetal and ketal reactions.
1. These alcohol addition reactions only occur with aldehydes and ketones.
2. Hemiacetal and hemiketal products can be identified by having one ether chain and one hydroxyl group attached to the original carbonyl carbon. Acetal and ketal groups have two ether groups attached to the original carbonyl carbon.
3. Acetal and hemiacetal groups will have a hydrogen attached to the original carbonyl carbon, where ketal and hemiketal groups will have carbon chains attached to the original carbonyl carbon (with no bound hydrogens).
Example Question #1412 : Mcat Biological Sciences
Which of the compounds below could not be made from an aldehyde reduction?
I, III, and IV
IV only
III only
II only
I only
II only
Of the choices given, all can be made from some type of aldehyde reduction except choice II. Choices I, III, and IV each have the terminal (or primary) alcohol that is characteristic of a former aldehyde. In contrast, choice II has a secondary alcohol, characteristic of a former ketone. In other words, if choice II was oxidized then the product would be a ketone, not an aldehyde.
Example Question #3 : Reactions
Acetaldehyde 

oxidized . . . carboxylic acid
reduced . . . alkane
reduced . . . alcohol
oxidized . . . amide
reduced . . . alkane
The correct answer is that the aldehyde is reduced to an alkane. In viewing the final product, we see that acetaldehyde would be reduced to ethane. The reaction of any aldehyde or ketone with hydrazine and the subsequent addition of base and heat will result in that aldehyde or ketone being reduced to an alkane, and is referred to as the Wolf-Kishner reaction. The Wolf-Kishner reagent is a commonly tested reducing agent.
Example Question #1421 : Mcat Biological Sciences
Compound B is dissolved in methylene chloride, and then treated with trifluoroacetic acid. Over the next thirty minutes, gas evolution was observed from the reaction mixture. What gas was being given off?

Treatment of a carboxylic acid with acid results in decarboxylation, and the evolution of 
All MCAT Biology Resources