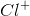All SAT II Chemistry Resources
Example Questions
Example Question #1 : Electron Configuration
Which of the following has the electron configuration of ![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex)
Possible Answers:
Correct answer:
Explanation:
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is 
Bhavi
Certified Tutor
Certified Tutor
Touro College, Master of Science, Biological and Physical Sciences. Touro College, Doctor of Medicine, Osteopathic Medicine (...
Brianna
Certified Tutor
Certified Tutor
Fairfield University, Bachelor of Science, Biology, General. National University of Ireland at Galway, Master of Science, Neu...
All SAT II Chemistry Resources
Popular Subjects
Algebra Tutors in Los Angeles, SAT Tutors in Denver, SSAT Tutors in Dallas Fort Worth, Spanish Tutors in Houston, English Tutors in New York City, Chemistry Tutors in Los Angeles, LSAT Tutors in Phoenix, Statistics Tutors in Philadelphia, ACT Tutors in Miami, Biology Tutors in Miami
Popular Courses & Classes
SSAT Courses & Classes in Boston, SAT Courses & Classes in New York City, GRE Courses & Classes in Houston, ISEE Courses & Classes in Philadelphia, SAT Courses & Classes in Atlanta, SSAT Courses & Classes in Washington DC, LSAT Courses & Classes in Dallas Fort Worth, ISEE Courses & Classes in Atlanta, ACT Courses & Classes in Denver, ACT Courses & Classes in New York City
Popular Test Prep
MCAT Test Prep in Los Angeles, MCAT Test Prep in Phoenix, SAT Test Prep in Phoenix, GRE Test Prep in Philadelphia, SSAT Test Prep in Dallas Fort Worth, ACT Test Prep in Denver, GMAT Test Prep in Los Angeles, ISEE Test Prep in Philadelphia, SSAT Test Prep in Washington DC, GRE Test Prep in New York City