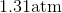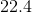All College Chemistry Resources
Example Questions
Example Question #1 : Gases
A gas does 


The correct answer is 



If a gas is expanding, it is doing work on the surroundings. This means that 

Example Question #1 : Properties Of Gases
The graph shows molecular velocities for four different molecules at the same temperature. Which molecule has the highest molar mass?

D
C
B
A
A
Recall that the kinetic theory of gases states that if molecules are at the same temperature, then the mass of the molecules will determine their velocities. In a mixture of gases at a given temperature, the heavier gases will, on average, travel slower than the lighter ones.
Thus, A must be the gas with the highest molar mass because it has the most molecules traveling at the slowest velocity.
Example Question #32 : Solutions, States Of Matter, And Thermochemistry
Which is not characteristic of gases?
Gases are easy to compress.
The pressure of a gas decreases as more gas is added to the container.
Gases expand to fill their containers.
Gases occupy more space than the liquids or solids that they form.
The pressure of a gas decreases as more gas is added to the container.
There is more free space between gas particles than there is between liquid particles, and more free space between liquid particles than there is between solid particles. This makes gases very easy to compress as compared to solids and liquids.
Both liquids and gases can expand to fill their containers because the individual particles can move past each other. This is not possible in a solid because the individual particles are rigidly packed.
Additionally, the volume of a liquid or solid can increase by approximately eight hundred times when it becomes a gas. This large change in volume can be harnessed to do work. For example, a steam engine works when water boils to form a gas (steam), which has a larger volume. Steam can thus escape from the container in which it was produced and perform work.
Lastly, the pressure of a gas increases as more gas is added to the container. Pressure is defined as the force exerted by the gas over a certain area. If more gas particles are added to a fixed area, they will exert a greater force over that area, increasing the overall pressure.
Example Question #1 : Properties Of Gases
The Haber process is a common reaction used in the industrial production of ammonia:
Which of the following would increase the production of NH3(g)?
Adding water
Increasing volume
Adding an inert gas
Decreasing volume
Increasing temperature
Decreasing volume
Let's first look at the affects of adding an inert gas such as 
Since He is on both sides of the equation, equilibrium is not affected.
Now let's look at the affects of heat. Because the reaction is exothermic, it can be rewritten as
Since heat is a product of the reaction, increasing temperature would shift the reaction to the left.
Finally, we know that changing the volume changes the internal pressure of the system. We know that the affect of this change is dependent on the number of moles in the gaseous phase.
In order to increase the production of NH3 (the side with the least number of moles), we need to increase the internal pressure of the system by decreasing the volume.
Decreasing the volume would increase the production of ammonia.
Example Question #41 : Solutions, States Of Matter, And Thermochemistry
One flask is at STP and another is at 
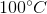
The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Example Question #1 : Non Ideal Gas Behavior
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.

Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Example Question #1 : Gas Laws
What is the pressure of a 


Recall the ideal gas law:

where 



and 
Since the question asks for pressure, rearrange the equation to solve for 
Plug in the given values. Remember that
Example Question #1 : Gases
A sample of gas at a constant temperature has an initial pressure of 


Since we are given the volume and the pressure of this sample of gas, we will need to use Boyle's Law, which states that the pressure and volume of a gas, at a constant temperature, are inversely related. As thus, we can then write the following equation:
Since all the answer choices are in units of atmospheres, we will need to convert the given units into atmospheres.
Plug in the given pressures and volume into the equation, and solve for 
Example Question #1 : Gas Laws
Consider the following chemical reaction:
How many grams of lithium is needed to react with 


Start by finding the number of moles of nitrogen gas by using the ideal gas law:
Rearrange the equation to solve for the variable 
In order to use the ideal gas law, the pressure must have units of atmospheres and the temperature must have units of Kelvin.
Convert the given pressure into atmospheres:
Now, substitute in all the given values in order to find the number of moles of nitrogen gas.
Next, use the stoichiometric ratio given by the chemical equation to find the number of moles of lithium needed to react completely with the nitrogen gas.
Finally, convert the number of moles of lithium to number of grams of lithium.
Example Question #4 : Gas Laws
What is the pressure exerted by 


Certified Tutor
Certified Tutor
All College Chemistry Resources

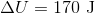
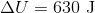
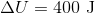









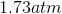
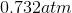

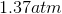


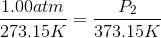


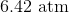




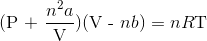
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)