All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #4 : Isomers
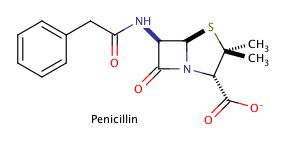
Shown above is the chemical structure for penicillin, a common prescription drug. How many chiral carbons does penicillin have?
Three
Zero
Two
One
Five
Three
The correct answer is three. The key to finding chiral carbons is to look for carbons that are attached to four different substituents. We can immediately eliminate any carbons that are involved in double bonds, or that have two hydrogens attached. Given this, we find that there are three chiral carbons. Note that carbon chains of varying content will qualify as different substituents, allowing chiral carbons to bond to two other carbons.
Example Question #211 : Gre Subject Test: Chemistry
Compounds that are mirror images of each other are called __________.
stereoisomers
enantiomers
diastereomers
conformers
enantiomers
Stereoisomers are isomers that differ in the orientation of atoms in space, but have the same bonding patterns and structures. Enantiomers are a specific class of stereoisomers that differ in orientation around a chiral center to create mirror image molecules.
Diastereomers are a type of stereoisomer that are not related through a reflection operation, and may differ at more than one chiral center. Conformers have the same structural formula, but different shapes due to bond rotation.
All GRE Subject Test: Chemistry Resources