All MCAT Biology Resources
Example Questions
Example Question #271 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following is not a lipid?
Prostaglandin
Linolenic acid
Galactocerebroside
Glycine
Glycine
Lipids are hydrophobic molecules that have low solubility in water and high solubility in nonpolar organic solvents. The following choices all describe lipid molecules, with the exception of glycine. Glycine is an amino acid and contains a carboxyl group (like fatty acid lipids), but also a amine group. These function groups make glycine hydrophilic and polar, unlike lipids.
Example Question #1 : Terpene Chemistry
Which of the following molecule is a type of terpene?





Terpenes are special classes of lipids that are derived from isoprene units. An isoprene unit’s molecular formula is 

The molecular formula 



Pentene is an alkene with the molecular formula 


Example Question #272 : Organic Chemistry, Biochemistry, And Metabolism
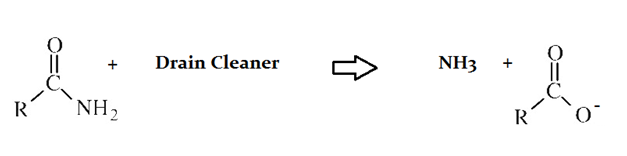
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The fats that are broken down by drain cleaners in the preceeding passage come predominately from oil secretions in skin and on hair. What is a main function of fats in the body?
To store energy in the form of free fatty acids
To create cytoskeletal elements
To lubricate joints
To store energy in the form of triacylglycerols
To dissolve lipids in the blood
To store energy in the form of triacylglycerols
Of the choices provided, only energy storage in the form of tricacylglycerols makes sense. Free fatty acids do not act as the main storage form for fats, but are used to create triacylglycerols.
Example Question #271 : Organic Chemistry, Biochemistry, And Metabolism
Which if the following lipids is responsible for the storing of energy in the body?
Triacylglycerols
Glycogen
Glycerophospholipids
Eicosanoids
Sterol lipids
Triacylglycerols
Triacylglycerols (triglycerides) are composed of three fatty acids and a glycerol backbone. Their primary function is to store energy. As a secondary function they can also provide thermal insulation for an organism.
Phospholipids (glycerophospholipids) are primarily used in the cell membrane, but can also be involved in signaling. Sterol lipids (steroids) are primarily used as hormones, or involved in cell signaling. Eicosanoids are a family of fatty acid involved in signaling.
Glycogen is used to store energy, but it is a carbohydrate glucose polymer. The question specifies that the answer must be a lipid.
Example Question #2 : Properties Of Lipids
Which of the following best describes steroid molecules?
Steroids are polar and can travel across the plasma membrane
Steroids are nonpolar and can travel across the plasma membrane
Steroids are nonpolar and cannot travel across the plasma membrane
Steroids are polar and cannot travel across the plasma membrane
Steroids are nonpolar and can travel across the plasma membrane
Steroids are a type of lipid. This means that steroids are nonpolar molecules, composed of primarily of hydrogen and carbon. Recall that the interior of the plasma membrane consists of the nonpolar tails of phospholipids. Since nonpolar molecules tend to dissolve in other nonpolar molecules, steroids will be able to traverse the interior (nonpolar phospholipid tail) of plasma membranes and cross into the cell.
Example Question #272 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following is false regarding steroids?
Steroids are the major component of plasma membranes
All steroids contain at least four rings
Steroid hormones bind to receptors on the nuclear membrane
Steroids and triglycerides are both classified as the same type of macromolecule
Steroids are the major component of plasma membranes
A steroid is a type of lipid molecule that is found extensively in living organisms. Recall that triglycerides are also a type of lipid; therefore, steroids and triglycerides are classified as the same type of macromolecule. One of the characteristics of steroids is that they have four cycloalkane rings. Three of the four rings are six-membered rings (cyclohexane) and one ring is a five-membered ring (cyclopentane). Steroids are found in several biomolecules, such as hormones. Some examples of steroid hormones include the sex hormones (estrogen, progesterone, and testosterone), aldosterone, and cortisol. Since they are nonpolar, steroid hormones can traverse the lipid bilayer and enter the cell; therefore, the steroid hormone receptors are found inside the cell (on the nuclear membrane).
A type of steroid, called cholesterol, can be found in plasma membranes but it is not the major component of the membrane. Recall that plasma membranes are primarily composed of a phospholipid bilayer; therefore, phospholipids (another type of lipid) are the major component of plasma membranes.
Example Question #1 : Properties Of Lipids
Which of the following will you most likely find in a steroid molecule?
Cyclohexane ring
Nitrogenous base
Phosphate group
Pentose sugar
Cyclohexane ring
Steroids are a type of lipid that are characterized by their four-ring molecular structure. The four rings consist of three six-membered rings and one five-membered ring. Recall that six-membered rings are called cyclohexanes and five-membered rings are called cyclopentanes; therefore, you will most likely find a cyclohexane in a steroid.
Phosphate groups, pentose sugars, and nitrogenous bases are found in nucleotides, which are monomers that make up nucleic acids. Steroids are a type of lipid; therefore, you will most likely not find these substances in a steroid.
Example Question #2 : Lipids
Given the same weight of each, from which macromolecule can the most heat be generated in a combustion reaction?
Saturated fat
Carbohydrate
Nucleic acid
Protein
Polyunsaturated fat
Saturated fat
The correct answer is saturated fat. Rather than attempting to draw each one of these macromolecules, one should view this conceptually. In a combustion reaction, heat is generated for every carbon-hydrogen bond that is oxidized. In other words, the higher the density of C-H bonds in a compound, the more potential energy it can store. Fats, which primarily consist of only carbon and hydrogen, will contain the most energy and subsequently generate the most heat when oxidized. This is the same reason why animals and plants store energy as fat molecules.
*Note: A polyunsaturated fat has more than one double bond between its carbons, which reduces the total number of C-H bonds.
Example Question #111 : Macromolecules
Sexually transmitted diseases are a common problem among young people in the United States. One of the more common diseases is caused by the bacterium Neisseria gonorrhoeae, which leads to inflammation and purulent discharge in the male and female reproductive tracts.
The bacterium has a number of systems to evade host defenses. Upon infection, it uses pili to adhere to host epithelium. The bacterium also uses an enzyme, gonococcal sialyltransferase, to transfer a sialyic acid residue to a gonococcal surface lipooligosaccharide (LOS). A depiction of this can be seen in Figure 1. The sialyic acid residue mimics the protective capsule found on other bacterial species.
Once infection is established, Neisseria preferentially infects columnar epithelial cells in the female reproductive tract, and leads to a loss of cilia on these cells. Damage to the reproductive tract can result in pelvic inflammatory disease, which can complicate pregnancies later in the life of the woman.

What is likely true of the lipid A found on the glucosamine molecule in Figure 1?
It is unable to anchor in the cell membrane due to its hydrophilicity
It is composed predominately of hydrogens and carbons
It is synthesized by ribosomes
It is derived from host proteins
It contains only nitrogen, carbon, and hydrogen
It is composed predominately of hydrogens and carbons
Lipids are hydrocarbons, and are the most energy-rich biological macromolecules due to their heavily reduced state. This reduced state is a function of the roughly equal electronegativity between carbon and hydrogen atoms.
Example Question #3 : Lipids
Water is the solvent in which all chemical reactions take place for living organisms. In addition, water has a number of critical characteristics that allows it to be invaluable to life as we know it.
Which of the following statements is FALSE concerning the properties of water?
Water's ability to form hydrogen bonds allow it to maintain a liquid state in nature.
Water plays a major role in the breakdown of macromolecules.
Water solvates hydrophobic molecules, such as fatty acids, and separates them.
Water's strong surface tension allows certain bugs to "stand" on the water's surface.
Water solvates hydrophobic molecules, such as fatty acids, and separates them.
Water is able to solvate hydrophilic compounds. Hydrophobic molecules, such as fatty acids, are typically aggregated together by water and can be separated.
Certified Tutor
All MCAT Biology Resources